推薦產品
等級
certified reference material
agency
BCR®
製造商/商標名
JRC
技術
HPLC: suitable
gas chromatography (GC): suitable
形式
neat
儲存溫度
2-8°C
SMILES 字串
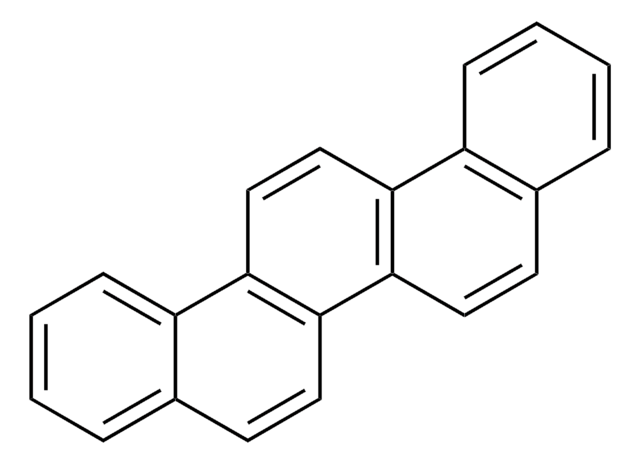
c1ccc2c(c1)ccc3cc4ccc5ccccc5c4cc23
InChI
1S/C22H14/c1-3-7-19-15(5-1)9-11-17-13-18-12-10-16-6-2-4-8-20(16)22(18)14-21(17)19/h1-14H
InChI 密鑰
KLIHYVJAYWCEDM-UHFFFAOYSA-N
分析報告
For more information please see:
BCR095
BCR095
法律資訊
BCR is a registered trademark of European Commission
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
W Baer-Dubowska et al.
Chemical research in toxicology, 8(2), 292-301 (1995-03-01)
The formation of deoxyribonucleoside adducts in mouse epidermis has been examined following topical application of [3H]dibenz[a,j]anthracene (DB[a,j]A) or by 32P-postlabeling following topical application of unlabeled DB[a,j]A, DB[a,j]A trans-3,4-diol or the anti- or syn-3,4-diol 1,2-epoxides. A single topical application of [3H]DB[a,j]A
Dibenz[a,j]anthracene.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, 32, 309-313 (1983-12-01)
S V Vulimiri et al.
Chemical research in toxicology, 12(1), 60-67 (1999-01-20)
The formation of DNA adducts in mouse epidermis has been examined following topical application of dibenz[a,j]anthracene (DB[a,j]A) and its metabolites, i.e., DB[a,j]A-3,4-diol, DB[a,j]A-3,4-10, 11-bis-diol, DB[a,j]A-3,4-8,9-bis-diol, 10-OH-DB[a,j]A-3,4-diol, or 11-OH-DB[a,j]A-3,4-diol, using a 32P-postlabeling assay. At initiating doses (400-1600 nmol), DB[a,j]A produced at
R V Nair et al.
Chemical research in toxicology, 4(1), 115-122 (1991-01-01)
Identification of various deoxyribonucleoside adducts formed in primary cultures of mouse keratinocytes exposed to dibenz[a,j]anthracene (DB[a,j]A) is presented. A preliminary analysis of the DNA adducts formed from 7-methyldibenz[a,j]anthracene (7MeDB[a,j]A) also is presented. Cultures of keratinocytes obtained from dorsal skins of
R V Nair et al.
Chemical research in toxicology, 5(4), 532-540 (1992-07-01)
The identification of several metabolites formed from dibenz[aj]anthracene (DB[aj]A) and 7-methyldibenz[aj]anthracene (7MeDB[aj]A) in primary cultures of mouse keratinocytes is presented. The metabolites were analyzed by coelution with known synthetic standards using high-pressure liquid chromatography. The metabolite identifications were further facilitated
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務