推薦產品
等級
derivatization grade ((chiral))
for chiral derivatization
品質等級
蒸汽壓力
0.5 mmHg ( 20 °C)
化驗
≥99.0% (sum of enantiomers, GC)
≥99.0%
形狀
liquid
光學活性
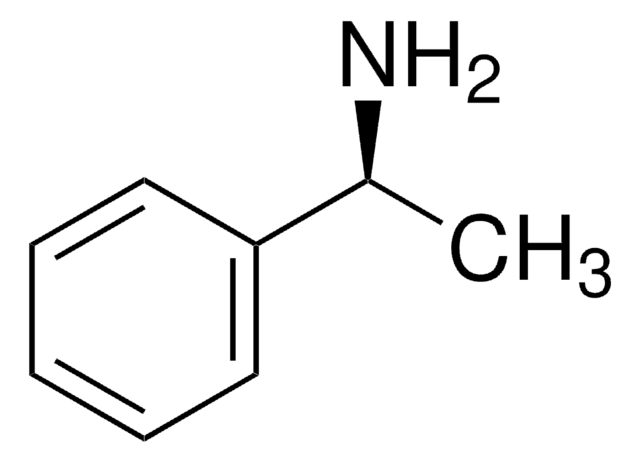
[α]20/D −30±1°, c = 10% in ethanol
光學純度
enantiomeric ratio: ≥99.5:0.5 (GC)
品質
LiChropur™
技術
HPLC: suitable
折射率
n20/D 1.526 (lit.)
n20/D 1.528
bp
187 °C (lit.)
密度
0.94 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
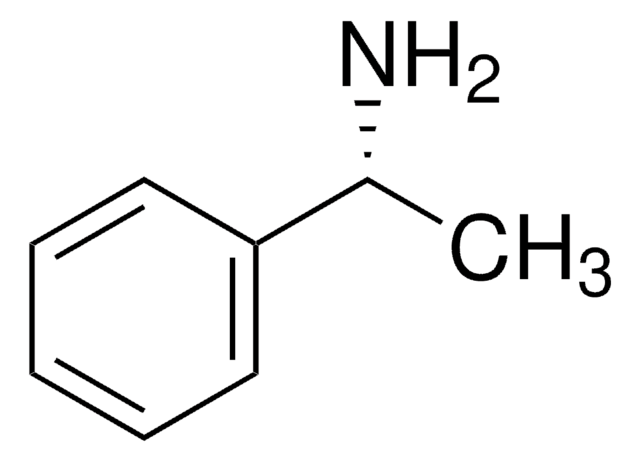
C[C@H](N)c1ccccc1
InChI
1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3/t7-/m0/s1
InChI 密鑰
RQEUFEKYXDPUSK-ZETCQYMHSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
(S)-(−)-α-Methylbenzylamine is a chiral derivatizing agent, which is employed for derivatizing enantiomers into diastereoisomers.
應用
(S)-(−)-α-Methylbenzylamine may be used as a chiral derivatizating reagent for the determination of acetyl-D-carnitine (D-AC) in acetyl-L-carnitine (L-AC) using high-performance liquid chromatographic (HPLC) enantioseparation method.
法律資訊
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Skin Corr. 1B
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
158.0 °F - closed cup
閃點(°C)
70 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Alejandra León et al.
Journal of natural products, 75(5), 859-864 (2012-05-12)
The enantiomeric lactams (-)-8, (+)-8, (+)-9, and (-)-9 were formed by the reaction of the dimeric phthalide rac-tokinolide B (rac-3) with (R)-(+)-α-methylbenzylamine and (S)-(-)-α-methylbenzylamine. The absolute configurations of compounds 8 and 9 were assigned by experimental and theoretically calculated electronic
Paul E Harrington et al.
Current medicinal chemistry, 14(28), 3027-3034 (2008-01-29)
The calcium sensing receptor (CaR) is a G protein-coupled receptor (GPCR) that plays a fundamental role in serum calcium homeostasis. The CaR is expressed on the chief cells of the parathyroid gland and is responsible for controlling the secretion of
Antoine Fadel et al.
The Journal of organic chemistry, 72(5), 1780-1784 (2007-01-30)
Enantiomerically pure (R)-(+)-pipecolic acid was synthesized in four steps and 42% overall yield starting from dihydropyran and (R)-alpha-methylbenzylamine. A general short strategy is also described for preparing (S)-proline (47.5% overall yield) and derivatives.
Carlos Fernandes et al.
The Journal of organic chemistry, 74(8), 3217-3220 (2009-03-17)
A short, convenient, gram scale protocol has been established to allow facile access to all four stereoisomers of 2-aminocyclobutanecarboxylic acid, each in enantiomerically pure form (ee >99%). Starting from the readily available cis racemate, the procedure combines efficient alpha-phenylethylamine derivative
Abraham R Martin et al.
Applied microbiology and biotechnology, 76(4), 843-851 (2007-06-22)
Enzyme immobilization often improves process economics, but changes in kinetic properties may also occur. The immobilization of a recombinant thermostable (S)-aminotransferase was made by entrapment on calcium alginate-3% (w/v)-and tested with (S)-(-)-(alpha)-methylbenzylamine for acetophenone production. The best immobilization results were
Chromatograms
HPLC Analysis of α-Methylbenzylamine Enantiomers (DANSYL Derivatives) on Astec® CYCLOBOND I 2000 DMP
application for HPLCapplication for HPLC我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務