推薦產品
等級
analytical standard
品質等級
產品線
VETRANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
clinical
形式
neat
儲存溫度
2-8°C
SMILES 字串
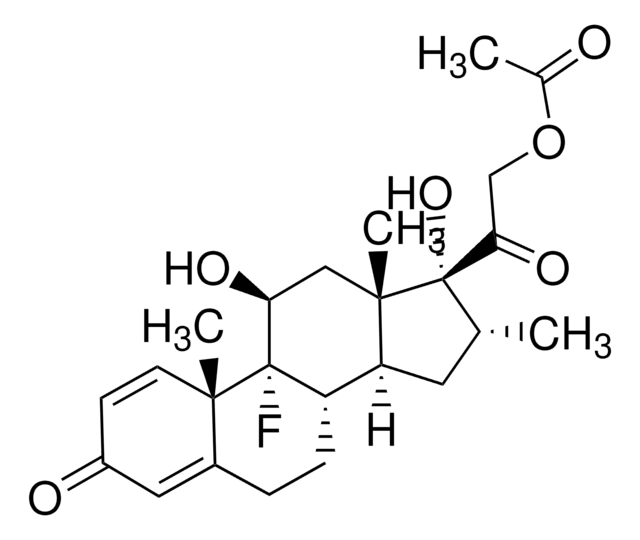
[H][C@@]12CCC3=CC(=O)C=C[C@]3(C)[C@@]1(F)[C@@H](O)C[C@@]4(C)[C@@]2([H])C[C@@H](C)[C@]4(O)C(=O)COC(C)=O
InChI
1S/C24H31FO6/c1-13-9-18-17-6-5-15-10-16(27)7-8-21(15,3)23(17,25)19(28)11-22(18,4)24(13,30)20(29)12-31-14(2)26/h7-8,10,13,17-19,28,30H,5-6,9,11-12H2,1-4H3/t13-,17+,18+,19+,21+,22+,23+,24+/m1/s1
InChI 密鑰
AKUJBENLRBOFTD-RPRRAYFGSA-N
基因資訊
human ... NR3C1(2908)
尋找類似的產品? 前往 產品比較指南
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
法律資訊
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
未找到適合的產品?
試用我們的產品選擇工具.
訊號詞
Danger
危險聲明
危險分類
Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Anti-inflammatory activity of injectable dexamethasone acetate-loaded nanostructured lipid carriers.
Xuefan Xu et al.
Drug delivery, 18(7), 485-492 (2011-06-23)
This work studied the intravenous injection formulation of nanostructured lipid carriers (NLCs) loaded with dexamethasone acetate (DA), a poorly water-soluble drug. The goal of this study was to design nanoparticles which could improve therapeutic efficacy of DA on inflammations. Based
Kam-Lun Ellis Hon et al.
The Journal of dermatological treatment, 19(4), 241-245 (2008-07-17)
Many parents purchase topical applications without knowing what they contain, and apply them liberally to their children with dermatological disorders. In one such case, an infant developed fever, diarrhea and a small ulcer near the right labia majora which was
T Lifshitz et al.
Ophthalmic surgery and lasers, 32(2), 159-161 (2001-04-13)
Retrocorneal membranes after penetrating keratoplasty (PKP) is a well known complications, resulting from unintentional retention of the host Descemet's membrane (DM), or donor DM detachment. We describe for the first time the formation of a retrocorneal inflammatory membrane that mimics
Sandra A L Moura et al.
Journal of pharmaceutical sciences, 100(7), 2886-2895 (2011-02-02)
Implants are defined as controlled sustained release delivery systems of therapeutic agents incorporated or dispersed into a polymeric carrier. These systems can be implanted in specific organs and delivered by the therapeutic agents at the target site to treat various
S L Fialho et al.
Current eye research, 31(6), 525-534 (2006-06-14)
The treatment of vitreoretinal diseases is limited and, nowadays, new drug delivery approaches have been reported in order to increase drug bioavailability. The objective of the current study was to determine the pharmacokinetic profile of a biodegradable dexamethasone acetate implant
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務