全部照片(1)
About This Item
經驗公式(希爾表示法):
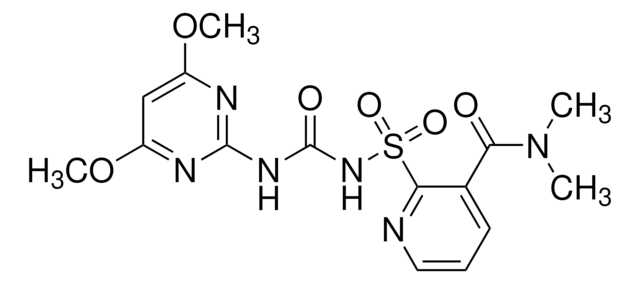
C14H17N5O7S2
CAS號碼:
分子量::
431.44
Beilstein:
7501778
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
analytical standard
品質等級
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
agriculture
environmental
形式
neat
SMILES 字串
CCS(=O)(=O)c1cccnc1S(=O)(=O)NC(=O)Nc2nc(OC)cc(OC)n2
InChI
1S/C14H17N5O7S2/c1-4-27(21,22)9-6-5-7-15-12(9)28(23,24)19-14(20)18-13-16-10(25-2)8-11(17-13)26-3/h5-8H,4H2,1-3H3,(H2,16,17,18,19,20)
InChI 密鑰
MEFOUWRMVYJCQC-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Rimsulfuron is a sulfonylurea herbicide primarily used to control a wide variety of perennial and annual grasses and some broadleaf weeds. The mode of action of rimsulfuron involves inhibition of acetolactate synthase (ALS), a key enzyme in the biosynthetic pathway of branched-chain amino acids.
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Rimsulfuron may be used as a reference standard for the determination of the analyte:
- In honey samples using a method based on an on-column liquid-liquid extraction (OCLLE) using diatomaceous earth as inert solid support and liquid chromatography (LC) coupled to mass spectrometry (MS) operating in tandem mode (MS/MS).
法律資訊
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Development and validation of a multi-residue method for pesticide determination in honey using on-column liquid- liquid extraction and liquid chromatography- tandem mass spectrometry.
Pirard C, et al.
Journal of Chromatography A, 1152(1-2), 116-123 (2007)
Basis of selectivity of the herbicide rimsulfuron in maize.
Koeppe MK, et al.
Pesticide Biochemistry and Physiology, 66(3), 170- 181 (2000)
Paola Ganugi et al.
Plant science : an international journal of experimental plant biology, 303, 110727-110727 (2021-01-26)
Herbicide application is a common procedure in agriculture, whose potentially adverse effects are assessed mainly with respect to weeds or in terms of residues and environmental impact. However, recent evidence has highlighted possible effects of pesticide treatments on plant metabolism
Shuo Wang et al.
Bulletin of environmental contamination and toxicology, 105(4), 602-606 (2020-09-27)
A method for simultaneous quantitation of rimsulfuron, quizalofop-P-ethyl and quizalofop-P in potato plant, soil and potato tuber samples was established. The mean recoveries of rimsulfuron, quizalofop-P-ethyl and quizalofop-P in different matrices spiked with them were 81.4%-101.1%, 76.1%-99.0% and 77.4%-106.4% with
Amit Paporisch et al.
Pesticide biochemistry and physiology, 138, 22-28 (2017-05-01)
Three sweet corn genotypes, two inbred lines (IBER001 and IBER002) and their hybrid (ER00X), differ in their phenotypic responses to several P450-metabolized herbicides, used in sweet corn, namely, foramsulfuron, iodosulfuron, rimsulfuron and tembotrione. Foramsulfuron is a sulfonylurea herbicide commonly formulated
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務