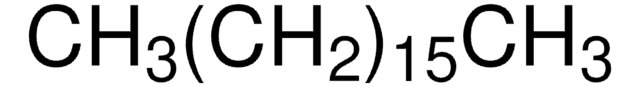全部照片(1)
About This Item
線性公式:
NH4Cl
CAS號碼:
分子量::
53.49
Beilstein:
4371014
EC號碼:
MDL號碼:
分類程式碼代碼:
12352300
eCl@ss:
38050207
PubChem物質ID:
NACRES:
NA.21
推薦產品
等級
ACS reagent
puriss. p.a.
品質等級
agency
USP/NF
reag. ISO
reag. Ph. Eur.
蒸汽密度
1.9 (vs air)
蒸汽壓力
1 mmHg ( 160.4 °C)
化驗
≥99.5%
形狀
crystalline powder
雜質
acidity or alkalinity, complies
bromides and iodides, complies
≤0.005% insoluble matter
pH值
4.5-5.5 (20 °C, 5%)
mp
340 °C (subl.) (lit.)
負離子痕跡
nitrate (NO3-): ≤5 mg/kg
phosphate (PO43-): ≤2 mg/kg
sulfate (SO42-): ≤20 mg/kg
正離子痕跡
As: ≤0.00005%
Ca: ≤0.0005%
Cu: ≤0.0002%
Fe: ≤0.0002%
K: ≤0.005%
Mg: ≤0.0005%
Na: ≤0.005%
Ni: ≤0.0001%
Pb: ≤0.0001%
Zn: ≤0.0002%
heavy metals: ≤5 mg/kg (by ICP-OES)
適合性
complies for appearance of solution
SMILES 字串
N.Cl
InChI
1S/ClH.H3N/h1H;1H3
InChI 密鑰
NLXLAEXVIDQMFP-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
Ammonium chloride can be used as a catalyst:
- To synthesize 3,4-dihydropyrimidin-2-(1H)-ones via solvent-free Biginelli condensation reaction.
- For the aldol condensation of carbonyl compounds.
- In the thia-Michael addition reaction of thiols to different α-enones.
- A promoter in the enantioselective hydrogenation of an unprotected β-enamine amides.
- A pH-adjuster in the preparation of waterborne glucose silicone resin.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Ammonium chloride-catalyzed one-pot synthesis of 3, 4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions
Shaabani A, et al.
Tetrahedron Letters, 44, 857-859 (2003)
Neutron diffraction study of the crystal structure of ammonium chloride.
Levy, Henri A., and S. W. Peterson.
Physical Review, 86(5), 766-766 (1952)
Barbara Nozière et al.
Physical chemistry chemical physics : PCCP, 12(15), 3864-3872 (2010-04-02)
In natural environments such as atmospheric aerosols, organic compounds coexist with inorganic salts but, until recently, were not thought to interact chemically. We have recently shown that inorganic ammonium ions, NH(4)(+), act as catalysts for acetal formation from glyoxal, a
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務