全部照片(2)
About This Item
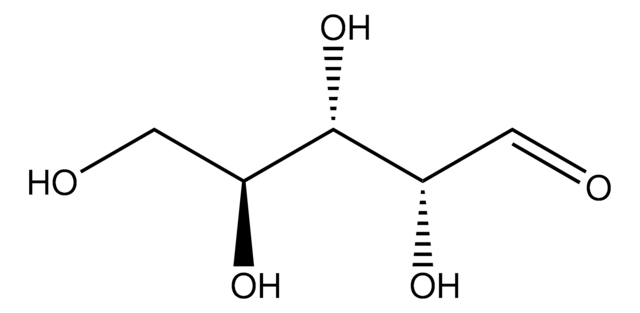
經驗公式(希爾表示法):
C5H10O5
CAS號碼:
分子量::
150.13
Beilstein:
1723079
EC號碼:
MDL號碼:
分類程式碼代碼:
41106212
PubChem物質ID:
NACRES:
NA.85
推薦產品
品質等級
化驗
≥99.0% (sum of enantiomers, HPLC)
≥99.0%
形狀
powder
光學活性
[α]20/D −104±2.0°, 24 hr, c = 10% in H2O
燃燒殘留物
≤0.1% (as SO4)
損耗
≤0.1% loss on drying, 20 °C (HV)
mp
162-164 °C (lit.)
負離子痕跡
chloride (Cl-): ≤50 mg/kg
sulfate (SO42-): ≤50 mg/kg
正離子痕跡
As: ≤0.1 mg/kg
Ca: ≤500 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤25 mg/kg
Fe: ≤17 mg/kg
K: ≤50 mg/kg
Mg: ≤10 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤15 mg/kg
應用
microbiology
SMILES 字串
O[C@@H]1COC(O)[C@@H](O)[C@@H]1O
InChI
1S/C5H10O5/c6-2-1-10-5(9)4(8)3(2)7/h2-9H,1H2/t2-,3-,4+,5?/m1/s1
InChI 密鑰
SRBFZHDQGSBBOR-ZRMNMSDTSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
D-Arabinose is a rare aldopentose, and is rarely utilized by enteric bacteria as a source of carbon and energy. It is also found in the aloins of the plant genus Aloe and as a constituent of the polysaccharide of the bacterial genus Mycobacterium. Some of the enteric bacteria like Escherichia coli K-12 can mutate to utilize D-arabinose.
應用
D-(-)-Arabinose has been used as an inducer of λ-RED recombinant gene expression.
生化/生理作用
D-阿拉伯糖是一种还原糖。它是一种 D-核糖的戊糖类似物,是组成分支杆菌细胞壁阿拉伯半乳聚糖的成分。也是酵母中 D-红抗坏血酸合成的底物。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
The Evolution of Metabolic Function.
Mortlock RP.
Science, 16-17 (1992)
Engineering complex biological systems in bacteria through recombinase-assisted genome engineering.
Santos CN and Yoshikuni Y2
Nature Protocols, 9, 1320-1336 (2014)
A Hasegawa et al.
Carbohydrate research, 52, 137-149 (1976-12-01)
Prumycin (1) and related compounds have been synthesized from benzyl 2-(benzyloxycarbonyl)amino-2-deoxy-5,6-O-isopropylidene-beta-D-glucofuranoside (4). Benzoylation of 4, followed by deisopropylidenation, gave benzyl 3-O-benzoyl-2-(benzyloxycarbonyl)amino-2-deoxy-beta-D-glucofuranoside (6), which was converted, via oxidative cleavage at C-5-C-6 and subsequent reduction, into the related benzyl beta-D-xylofuranoside derivative (7).
Nobuhiro Yamagata et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(2), 578-583 (2014-12-31)
Drosophila melanogaster can acquire a stable appetitive olfactory memory when the presentation of a sugar reward and an odor are paired. However, the neuronal mechanisms by which a single training induces long-term memory are poorly understood. Here we show that
Agustina Llanos et al.
Microbial cell factories, 18(1), 14-14 (2019-01-30)
Research on filamentous fungi emphasized the remarkable redundancy in genes encoding hydrolytic enzymes, the similarities but also the large differences in their expression, especially through the role of the XlnR/XYR1 transcriptional activator. The purpose of this study was to evaluate
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務