全部照片(1)
About This Item
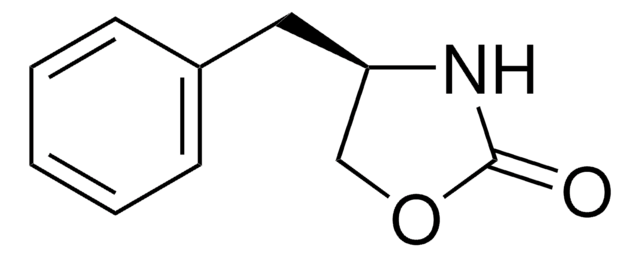
經驗公式(希爾表示法):
C9H9NOS
CAS號碼:
分子量::
179.24
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
≥98.0%
光學活性
[α]/D -75±5°, c = 0.2 in chloroform
光學純度
enantiomeric ratio: 97.0:3.0 (LC)
SMILES 字串
S=C1N[C@@H](CO1)c2ccccc2
InChI
1S/C9H9NOS/c12-9-10-8(6-11-9)7-4-2-1-3-5-7/h1-5,8H,6H2,(H,10,12)/t8-/m0/s1
InChI 密鑰
LVIJIGQKFDZTNC-QMMMGPOBSA-N
應用
具有高度选择性和有效性的手性助剂,与二异丁基氢化铝发生还原裂解反应可直接还原为相应的醛和手性助剂。
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Velazquez. F.; Olivo, H. F.
Current Organic Chemistry, 6, 303-303 (2002)
M T Crimmins et al.
Organic letters, 2(6), 775-777 (2001-02-07)
[formula: see text] Asymmetric aldol additions using chlorotitanium enolates of thiazolidinethione propionates proceed with high diastereoselectivity for the "Evans" or "non-Evans" syn product depending on the nature and amount of the base used. With (-)-sparteine as the base, selectivities of
文章
The asymmetric aldol reaction mediated by chiral auxiliaries is considered to be one of the most important methods for asymmetric C-C bond formation.
The asymmetric aldol reaction mediated by chiral auxiliaries is considered to be one of the most important methods for asymmetric C-C bond formation.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務