MSP010056
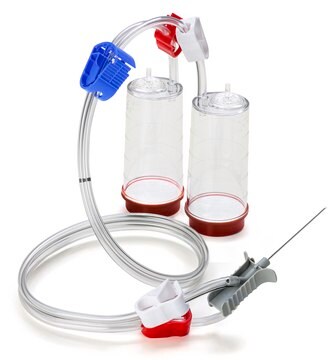
Steritest® NEO Device
For liquids in large vials with septa. Blue base canister with vented needle adapter. Single packed.
同義詞:
Blue Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
登入查看組織和合約定價
全部照片(1)
About This Item
分類程式碼代碼:
23151818
eCl@ss:
32014001
NACRES:
NB.24
推薦產品
材料
blue base (canister)
mixed cellulose esters (MCE) (for membrane filter)
plain filter
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
品質等級
agency
EP (2.6.1)
JP (4.06)
USP (71)
無菌
sterile; γ-irradiated
製造商/商標名
Steritest®
包裝
pkg of 10 blisters per box, Single packed
參數
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
顏色
blue Canister Base
基質
MF-Millipore™
孔徑
0.45 μm pore size
輸入
pharmaceutical(s)
應用
pharmaceutical
sterility testing
運輸包裝
ambient
一般說明
Device configuration: 2 canisters
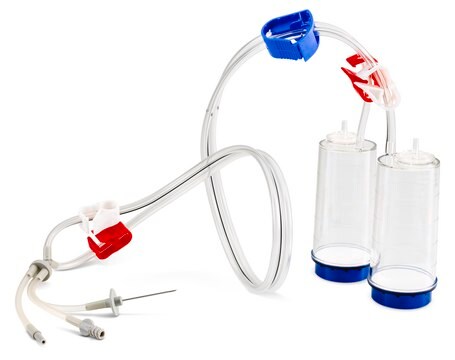
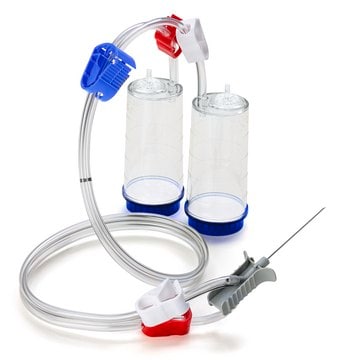
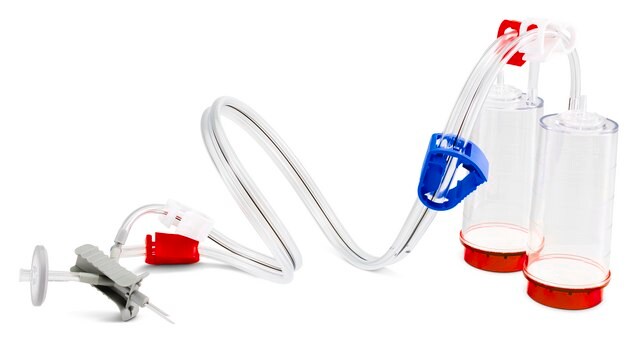
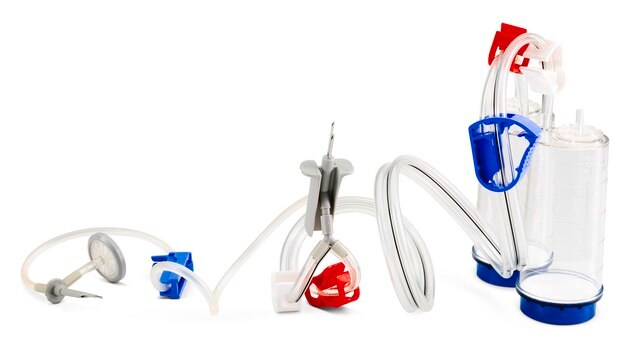
Steritest® NEO is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system minimizes false positives and offers the highest levels of quality and reliability. This device ensures that pharmaceutical products are never exposed to the environment during the testing process. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. A vented needle adapter vents and transfers the test product from large volume containers with a septum to the Steritest® NEO devices. The blue canister base indicates mixed esters of cellulose membrane. This membrane provides an optimal filtration flow rate for standard products.
Steritest® NEO is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system minimizes false positives and offers the highest levels of quality and reliability. This device ensures that pharmaceutical products are never exposed to the environment during the testing process. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. A vented needle adapter vents and transfers the test product from large volume containers with a septum to the Steritest® NEO devices. The blue canister base indicates mixed esters of cellulose membrane. This membrane provides an optimal filtration flow rate for standard products.
應用
The Steritest® NEO Device for liquids in vials is used for sterility testing of large volume parenterals and synthetic drugs without antimicrobial activity.
特點和優勢
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
- Smarter workflow
- Completely closed set up
- Consistent performance
- New tubing disconnection tool
包裝
Pack of 10 single packed blisters per box
法律資訊
MF-Millipore is a trademark of Merck KGaA, Darmstadt, Germany
STERITEST is a registered trademark of Merck KGaA, Darmstadt, Germany
VELAX is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務