推薦產品
品質等級
產品線
Novabiochem®
化驗
≥97.0% (acidimetric)
≥98% (TLC)
≥98.0% (HPLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
mp
95 °C (decomposes)
應用
peptide synthesis
官能基
carboxylic acid
儲存溫度
2-30°C
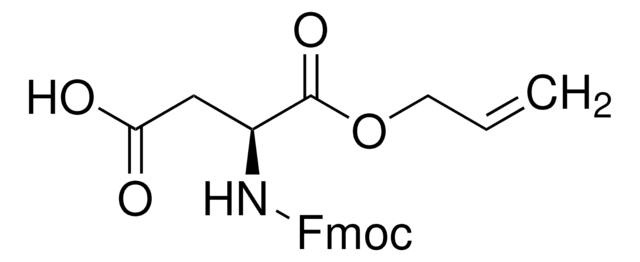
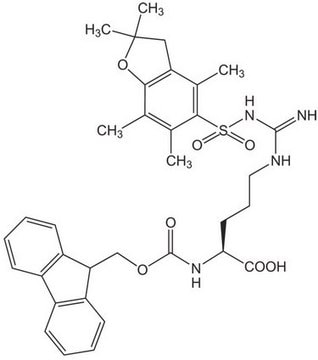
SMILES 字串
N([C@@H](CC(=O)O)C(=O)OCC=C)C(=O)OCC1c2c(cccc2)c3c1cccc3
InChI
1S/C22H21NO6/c1-2-11-28-21(26)19(12-20(24)25)23-22(27)29-13-18-16-9-5-3-7-14(16)15-8-4-6-10-17(15)18/h2-10,18-19H,1,11-13H2,(H,23,27)(H,24,25)/t19-/m0/s1
InChI 密鑰
ZJMVIWUCCRKNHY-IBGZPJMESA-N
一般說明
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] W. Bannwarth, et al. (1992) Tetrahedron Lett., 33, 4557.
[2] F. Albericio, et al. (1993) Tetrahedron Lett., 34, 1549.
[3] J. Eichler, et al. (1994) Pept. Res., 7, 300.
[4] J. Eichler, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1994, pp. 461.
[5] J. Eichler, et al. in ′Solid Phase Synthesis & Combinatorial Libraries, 4th International Symposium′, R. Epton (Eds), Mayflower Scientific Ltd., Birmingham, 1996, pp. 201.
[6] S. A. Kates, et al. in ′Peptides, Chemistry, Structure & Biology, Proc. 13th American Peptide Symposium′, ESCOM, Leiden, 1994, pp. 113.
聯結
分析報告
Appearance of substance (visual): powder
Identity (IR): conforms
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(CMA2)): ≥ 98 %
Purity (TLC(157A)): ≥ 98 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 97.0 %
Water (K. F.): ≤ 1.5 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
文章
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務