513100
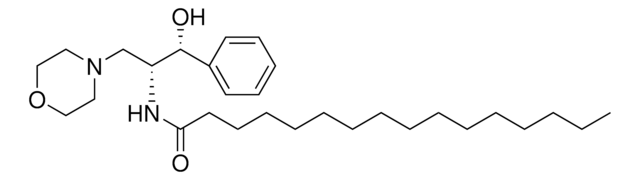
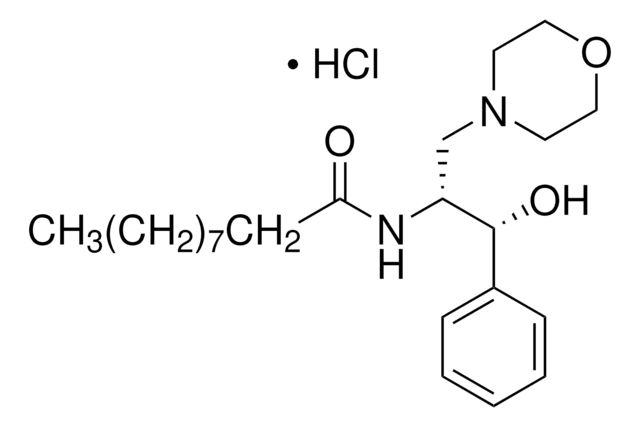
DL-threo-PDMP, Hydrochloride
PDMP closely resembles the natural sphingolipid substrate of brain glucosyltransferase and acts as a potent and competitive inhibitor of this enzyme.
同義詞:
DL-threo-PDMP, Hydrochloride, 1-Phenyl-2-decanoylamino-3-morpholino-1-propanol, HCl
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C23H38N2O3 · xHCl
CAS號碼:
分子量::
390.56 (free base basis)
MDL號碼:
分類程式碼代碼:
12352200
NACRES:
NA.77
推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
顏色
white
溶解度
DMSO: soluble
ethanol: soluble
methanol: soluble
運輸包裝
ambient
儲存溫度
−20°C
SMILES 字串
Cl.N2(CCOCC2)C[C@@H](NC(=O)CCCCCCCCC)[C@H](O)c1ccccc1
InChI
1S/C23H38N2O3.ClH/c1-2-3-4-5-6-7-11-14-22(26)24-21(19-25-15-17-28-18-16-25)23(27)20-12-9-8-10-13-20;/h8-10,12-13,21,23,27H,2-7,11,14-19H2,1H3,(H,24,26);1H/t21-,23-;/m1./s1
InChI 密鑰
HVJHJOYQTSEKPK-BLDCTAJRSA-N
一般說明
PDMP closely resembles the natural sphingolipid substrate of brain glucosyltransferase and acts as a potent and competitive inhibitor of this enzyme. Blocks the outgrowth of neurites and inhibits glycolipid synthesis in cultured NIH/3T3 cells.
生化/生理作用
Cell permeable: no
Primary Target
glycolipid synthesis in cultured NIH/3T3 cells
glycolipid synthesis in cultured NIH/3T3 cells
Product does not compete with ATP.
Reversible: no
警告
Toxicity: Irritant (B)
其他說明
Rani, C.S., et al. 1995. J. Biol. Chem. 270, 2859.
Rosenwald, A.G. 1992. Biochemistry31, 3581.
Inokuchi, J., and Radin, N.S. 1987. J. Lipid Res.28, 565.
Vunnam, R.R., and Radin, N.S. 1980. Chem. Phys. Lipids26, 265.
Rosenwald, A.G. 1992. Biochemistry31, 3581.
Inokuchi, J., and Radin, N.S. 1987. J. Lipid Res.28, 565.
Vunnam, R.R., and Radin, N.S. 1980. Chem. Phys. Lipids26, 265.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務