About This Item
meets purity specifications of JECFA
推薦產品
生物源
synthetic
品質等級
等級
FG
Fragrance grade
Halal
Kosher
agency
follows IFRA guidelines
meets purity specifications of JECFA
法律遵循
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 182.60
蒸汽壓力
0.17 mmHg ( 25 °C)
化驗
≥97%
成份
contains EU 1223/2009 restricted linalool
折射率
n20/D 1.462 (lit.)
bp
194-197 °C/720 mmHg (lit.)
溶解度
ethanol: soluble 1ml/4ml, clear, colorless (60% ethanol)
密度
0.87 g/mL at 25 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
香料過敏原
linalool
感官的
lemon; orange; floral; sweet
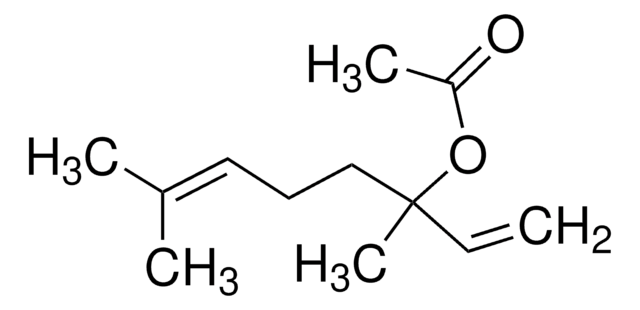
SMILES 字串
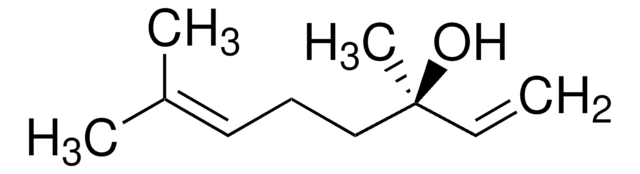
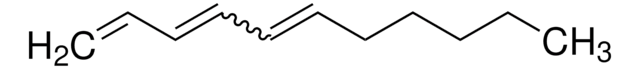
C\C(C)=C\CCC(C)(O)C=C
InChI
1S/C10H18O/c1-5-10(4,11)8-6-7-9(2)3/h5,7,11H,1,6,8H2,2-4H3
InChI 密鑰
CDOSHBSSFJOMGT-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
171.0 °F - Pensky-Martens closed cup
閃點(°C)
77.2 °C - Pensky-Martens closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| W263508-20KG-K | 4061837800399 |
| W263508-1KG-K | 4061837800382 |
| W263508-SAMPLE-K | 4061837800405 |
| W263508-9KG-K | 4061837515743 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務