全部照片(1)
About This Item
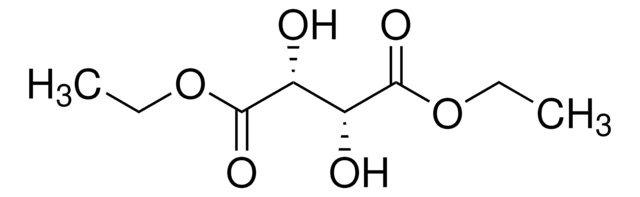
線性公式:
[-CH(OH)CO2C2H5]2
CAS號碼:
分子量::
206.19
FEMA號碼:
2378
Beilstein:
1727145
EC號碼:
歐洲委員會號碼:
440
MDL號碼:
分類程式碼代碼:
12164502
PubChem物質ID:
Flavis號碼:
9.446
NACRES:
NA.21
感官的:
fruity; wine-like
等級:
FG
Fragrance grade
Kosher
Fragrance grade
Kosher
生物源:
synthetic
agency:
follows IFRA guidelines
meets purity specifications of JECFA
meets purity specifications of JECFA
食物過敏原:
no known allergens
推薦產品
生物源
synthetic
品質等級
等級
FG
Fragrance grade
Kosher
agency
follows IFRA guidelines
meets purity specifications of JECFA
法律遵循
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
化驗
≥99%
光學活性
[α]20/D +8.5°, neat
折射率
n20/D 1.446 (lit.)
bp
280 °C (lit.)
密度
1.204 g/mL at 25 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
香料過敏原
no known allergens
感官的
fruity; wine-like
SMILES 字串
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
InChI 密鑰
YSAVZVORKRDODB-PHDIDXHHSA-N
尋找類似的產品? 前往 產品比較指南
應用
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
199.4 °F - closed cup
閃點(°C)
93 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Aman Ullah et al.
Biomacromolecules, 12(10), 3826-3832 (2011-09-06)
Poultry feather quills have been extruded in a twin screw extruder with sodium sulfite treatment as a reducing agent. The effect of four different plasticizers (ethylene glycol, propylene glycol, glycerol, and diethyl tartrate) on the thermoplastic properties was then investigated.
Total synthesis of the light-harvesting carotenoid peridinin.
Thomas Olpp et al.
Angewandte Chemie (International ed. in English), 45(24), 4023-4027 (2006-05-10)
Jiang Weng et al.
The Journal of organic chemistry, 75(9), 3125-3128 (2010-04-17)
A short and practical synthesis of oseltamivir was accomplished in 11 steps from inexpensive and abundant diethyl D-tartrate starting material. This azide-free route featured an asymmetric aza-Henry reaction and a domino nitro-Michael/Horner-Wadsworth-Emmons (HWE) reaction as the key steps to construct
Naoto Hama et al.
Organic letters, 13(4), 616-619 (2011-01-05)
A first total synthesis of broussonetine F from diethyl L-tartrate was achieved. The cornerstone of our synthesis was an orthoamide Overman rearrangement, which provided an allylic amino alcohol with complete diastereoselectivity.
Two-chiral component microemulsion EKC - chiral surfactant and chiral oil. Part 2: diethyl tartrate.
Kimberly A Kahle et al.
Electrophoresis, 28(15), 2644-2657 (2007-06-29)
In this second study on dual-chirality microemulsions containing a chiral surfactant and a chiral oil, a less hydrophobic and lower interfacial tension chiral oil, diethyl tartrate, is employed (Part 1, Foley, J. P. et al.., Electrophoresis, DOI: 10.1002/elps.200600551). Six stereochemical
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務