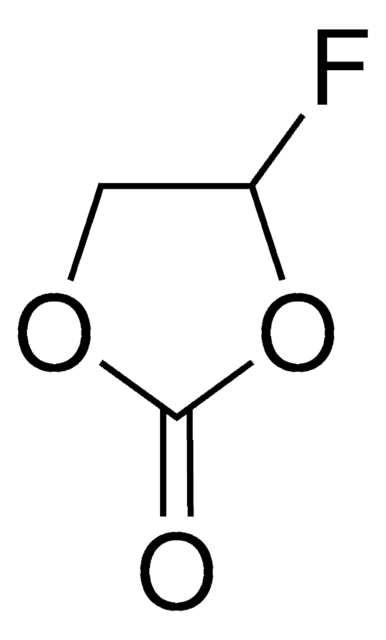
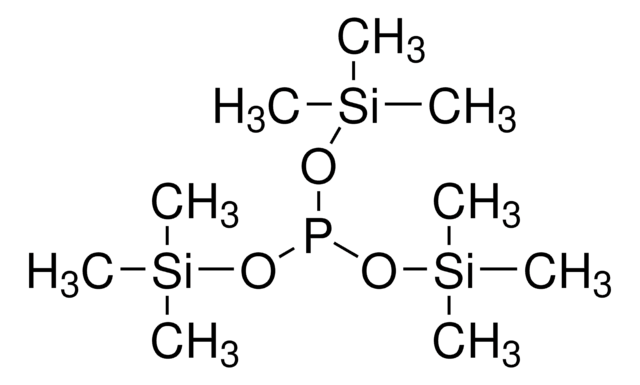
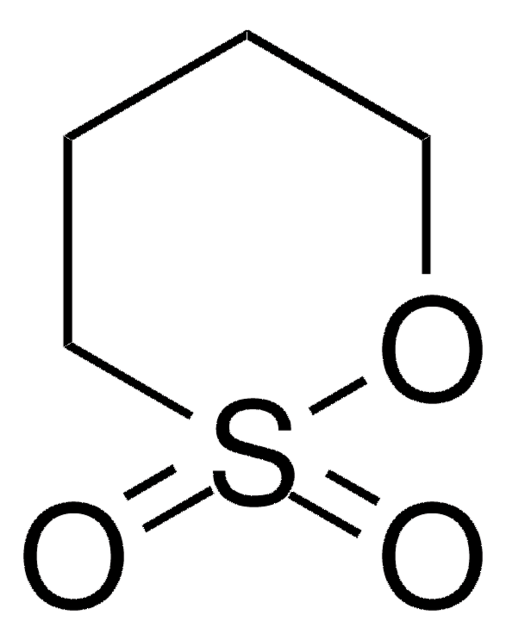
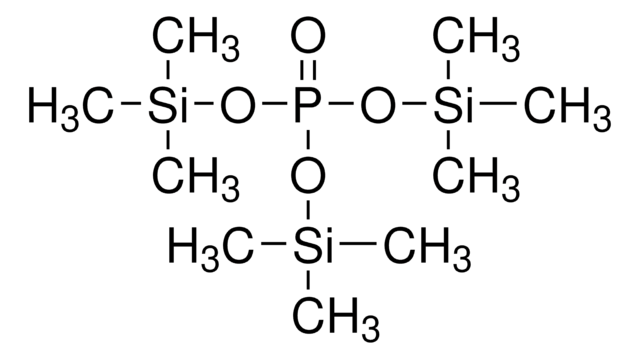
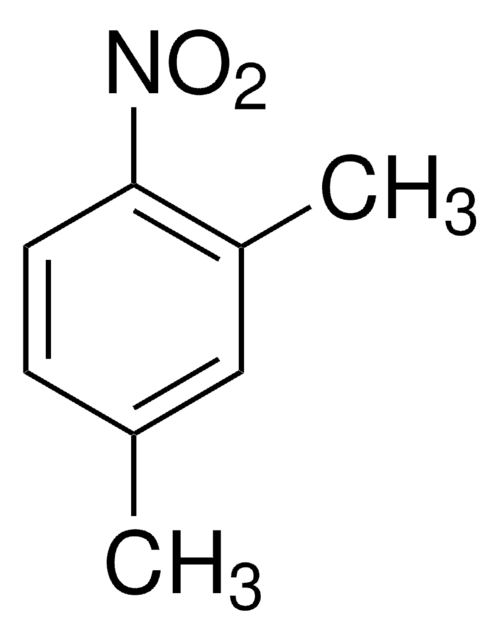
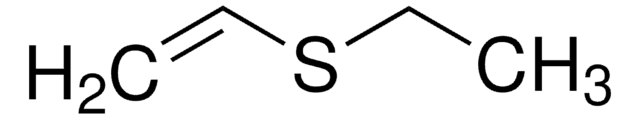推薦產品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.445 (lit.)
bp
159.1 °C (lit.)
密度
1.426 g/mL at 25 °C (lit.)
SMILES 字串
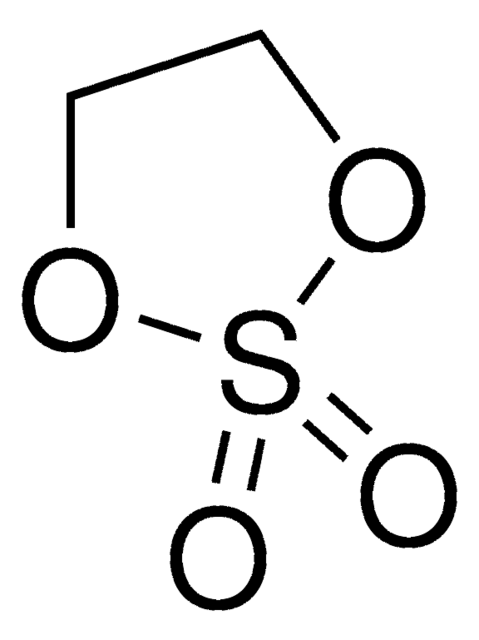
O=S1OCCO1
InChI
1S/C2H4O3S/c3-6-4-1-2-5-6/h1-2H2
InChI 密鑰
WDXYVJKNSMILOQ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
197.1 °F
閃點(°C)
91.7 °C
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Joseph P O'Shea et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 207-216 (2015-07-29)
Novel formulations that overcome the solubility limitations of poorly water soluble drugs (PWSD) are becoming ever more critical to a drug development process inundated with these compounds. There is a clear need for developing bio-enabling formulation approaches to improve oral
Dharmendra K Yadav et al.
AAPS PharmSciTech, 16(4), 855-864 (2015-01-15)
The objective of this study was to develop novel docetaxel phospholipid nanoparticles (NDPNs) for intravenous administration. Modified solvent diffusion-evaporation method was adopted in the NDPN preparation. Central composite design (CCD) was employed in the optimization of the critical formulation factor
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(6), 1734-1746 (2014-04-18)
The current study determined the extent to which the desorption of lipid-based formulations (LBFs) from a mesoporous magnesium aluminometasilicate (Neusilin®-US2) carrier is governed by drug properties, LBF composition, and LBF-to-adsorbent ratio. A secondary objective was to evaluate the impact of
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(8), 2441-2455 (2014-07-06)
The Lipid Formulation Classification System Consortium looks to develop standardized in vitro tests and to generate much-needed performance criteria for lipid-based formulations (LBFs). This article highlights the value of performing a second, more stressful digestion test to identify LBFs near
Mette U Anby et al.
Pharmaceutical research, 31(6), 1536-1552 (2014-01-31)
To explore the possibility that age-related changes in physiology may result in differences in drug bioavailability after oral administration of lipid based formulations of danazol. Danazol absorption from lipid formulations with increasing drug load was examined in younger (9 months)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務