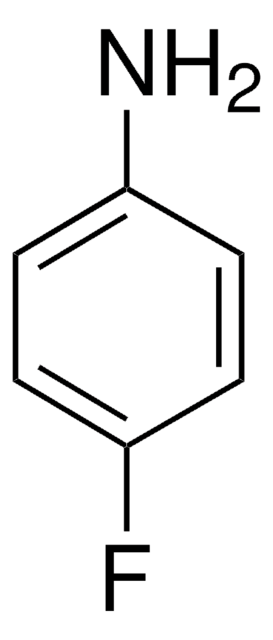
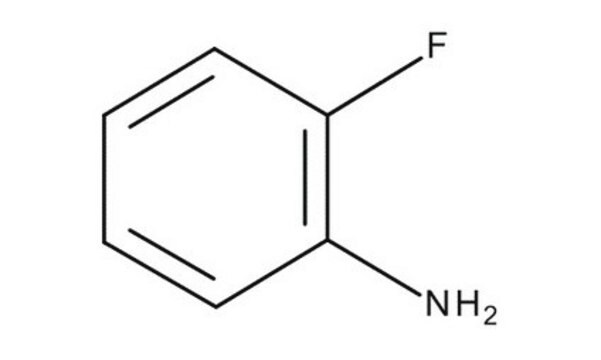
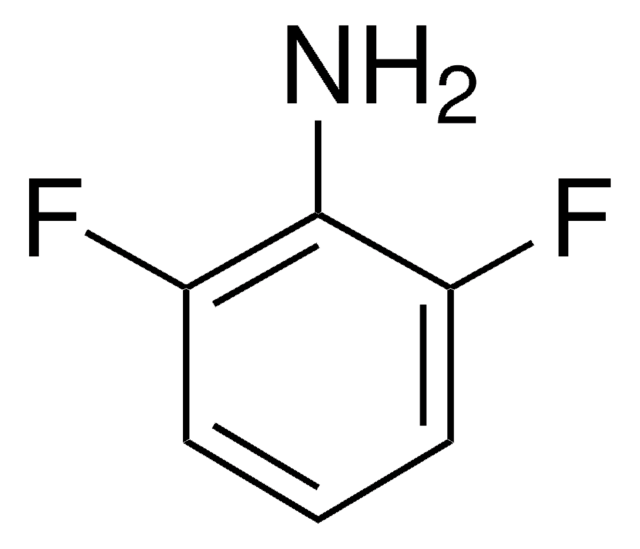
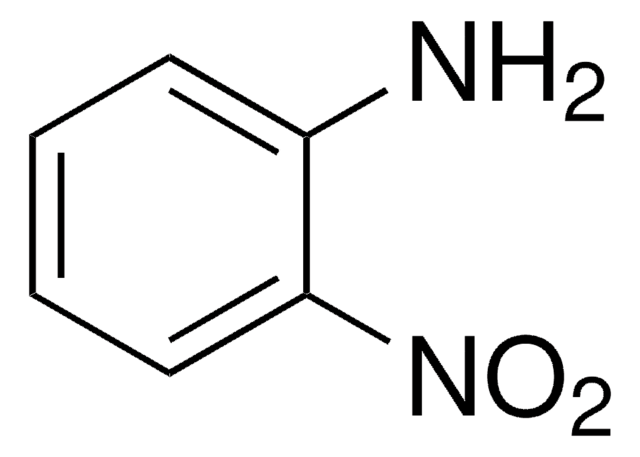
推薦產品
化驗
≥99%
折射率
n20/D 1.544 (lit.)
bp
182-183 °C (lit.)
mp
−29 °C (lit.)
密度
1.151 g/mL at 25 °C (lit.)
SMILES 字串
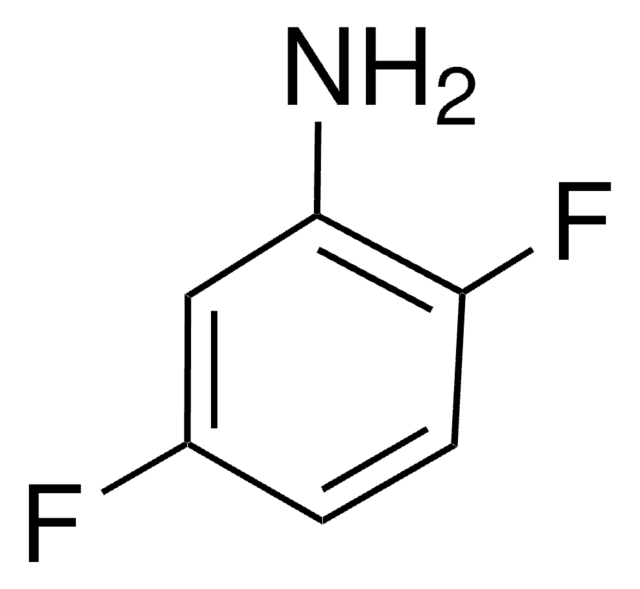
Nc1ccccc1F
InChI
1S/C6H6FN/c7-5-3-1-2-4-6(5)8/h1-4H,8H2
InChI 密鑰
FTZQXOJYPFINKJ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
生化/生理作用
由于人体暴露在制造中,异生素化合物 2-氟苯胺的代谢和排泄是重要的。发现它非常有效地代谢,主要是通过 4-羟基化,随后形成硫酸盐或葡糖苷酸。还观察到 N-乙酰化。至少 80% 的剂量在 24 小时内从尿液中排出。2-氟苯胺通过 4-羟基化和随后的对苯醌亚胺形成发挥其肾毒性作用。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1C - STOT RE 2
標靶器官
Blood,hematopoietic system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
140.0 °F - closed cup
閃點(°C)
60 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
A L Sharma et al.
Applied biochemistry and biotechnology, 96(1-3), 155-165 (2002-01-11)
Poly(2-fluoroaniline) was prepared by both chemical and electrochemical polymerization in acidic medium. Characterization of poly(2-fluoroaniline) was accomplished experimentally using ultraviolet-visible, Fourier transform infrared, differential scanning calorimetry, thermal gravimetric analysis, and X-ray diffraction techniques, respectively. Scanning electron microscopy studies revealed globular
M Arivazhagan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 668-676 (2012-08-14)
The Fourier transform infrared (FT-IR) and Fourier transform Raman (FT-Raman) spectra of 4-chloro-2-fluoroaniline (CFA) have been recorded and analyzed. The equilibrium geometry, bonding features and harmonic vibrational frequencies have been investigated with the help of ab initio and density functional
J Vervoort et al.
NMR in biomedicine, 4(6), 255-261 (1991-12-01)
The present study describes results from an in vivo 19F NMR study on rats exposed to the xenobiotic compound 2-fluoroaniline. Qualitative pharmacokinetics and the biotransformation of 2-fluoroaniline were studied after exposure to 50 mg/kg body wt 2-fluoroaniline. Accumulation and elimination
Suria Jahan et al.
Transfusion, 60(4), 769-778 (2020-03-19)
Platelet engraftment following cord blood (CB) transplantation remains a significant hurdle to this day. The uncontrolled growth of ice, a process referred to as ice recrystallization, is one of several mechanisms that lead to cell loss and decreased potency during
M A Mori et al.
Xenobiotica; the fate of foreign compounds in biological systems, 20(7), 653-656 (1990-07-01)
1. p-Hydroxymephentermine (p-hydroxy-MP) and p-hydroxyphentermine (p-hydroxy-Ph) were isolated as hydrochlorides from urine of male Wistar rats repeatedly dosed with mephentermine (MP). In addition, p-hydroxy-Ph was isolated as the hydrochloride from urine of the rats dosed with phentermine (Ph). 2. These
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務