推薦產品
蒸汽壓力
0.1 mmHg ( 100 °C)
品質等級
化驗
99%
形狀
crystals
bp
108 °C/0.15 mmHg (lit.)
mp
64-70 °C (lit.)
溶解度
H2O: 50 mg/mL, clear, colorless to very faintly yellow
SMILES 字串
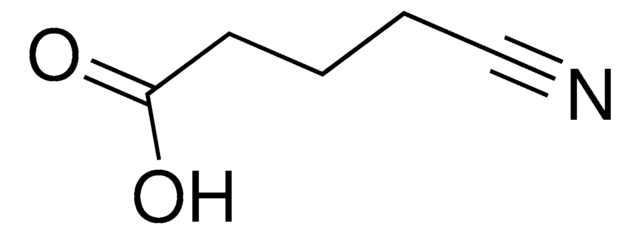
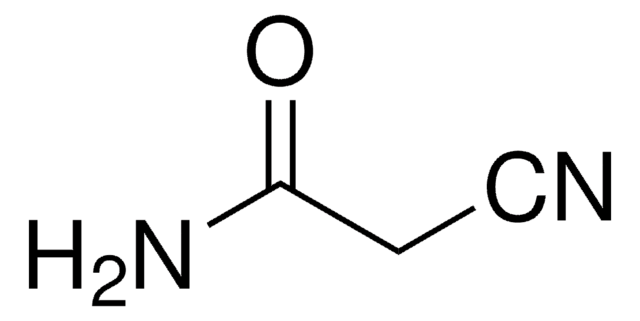
OC(=O)CC#N
InChI
1S/C3H3NO2/c4-2-1-3(5)6/h1H2,(H,5,6)
InChI 密鑰
MLIREBYILWEBDM-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
氰基乙酸可用作以下反应的试剂:
- 与乙酸酐一起用于各种吡咯、吲哚和苯胺衍生物的氰基乙酰化(cyanoacetylation)。它也可用于环化、香豆素和其他杂环的合成等其他反应中 。通过Knoevenagel缩合反应制备可作为MT3受体配体的5-乙酰氨基取代褪黑素衍生物的全合成过程的关键中间体。
- 通过Ugi加成物与氰基乙酸和芳香醛反应合成氨基吡咯啉酮(aminopyrrolinone )衍生物。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
安全危害
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
224.6 °F - closed cup
閃點(°C)
107 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
The Ugi Reaction of Cyanoacetic Acid as a Route to Tetramic Acid Derivatives
Alvarez-Rodriguez NV, et al.
Synlett, 26(16), 2253-2256 (2015)
Cyanoacetylation of indoles, pyrroles and aromatic amines with the combination cyanoacetic acid and acetic anhydride
Slaett J, et al.
Synthesis, 2004(16), 2760-2765 (2004)
Tian-Yu Liu et al.
Organic & biomolecular chemistry, 4(11), 2097-2099 (2006-05-27)
The bifunctional thiourea-tertiary amine derivatives of simple chiral diamines serve as highly enantioselective catalysts for the Michael addition of alpha-substituted cyanoacetates to vinyl sulfones, giving an efficient protocol for the construction of an all-carbon substituted quaternary stereocentre.
Isocyanoacetate derivatives: synthesis, reactivity, and application.
Anton V Gulevich et al.
Chemical reviews, 110(9), 5235-5331 (2010-07-09)
S Kotha et al.
Amino acids, 32(3), 387-394 (2006-10-13)
Two synthetic routes to bis-armed-alpha-amino acid derivatives are described. The first route involves alkylation of dibromo derivatives with ethyl isocyanoacetate under phase-transfer catalysis (PTC) conditions. The second route uses a palladium-mediated Suzuki-Miyaura cross-coupling reaction between a DL-4-boronophenylalanine derivative and aromatic
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務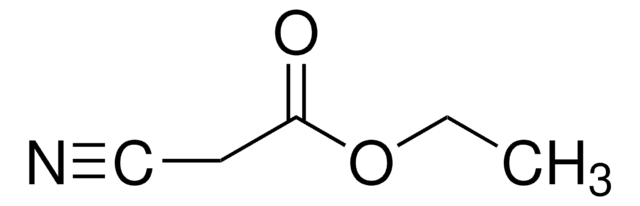


![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)