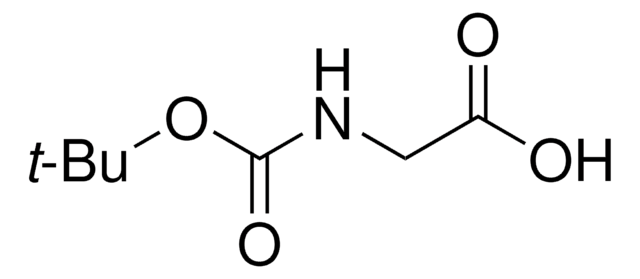
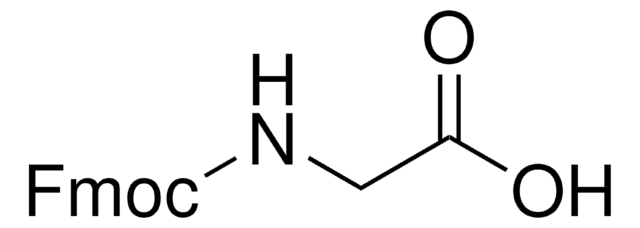
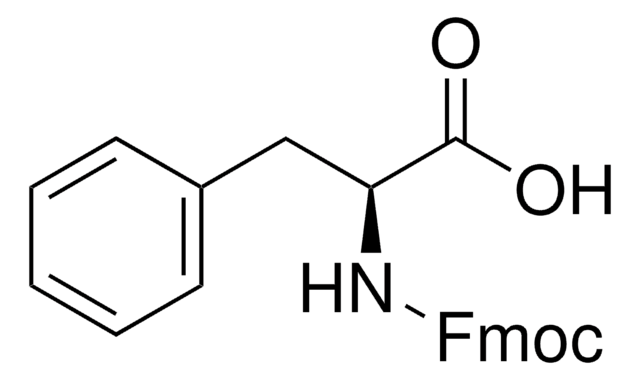
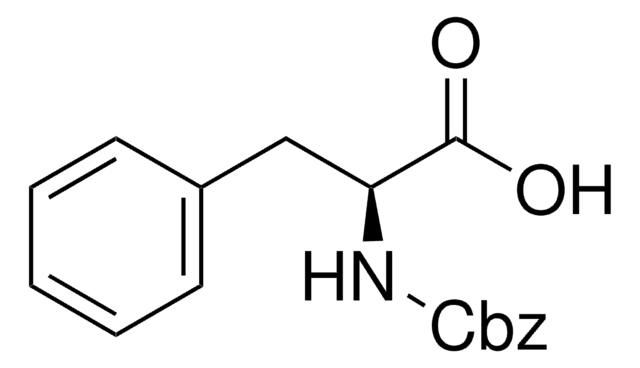
推薦產品
化驗
99%
形狀
powder
反應適用性
reaction type: solution phase peptide synthesis
顏色
beige
mp
118-122 °C (lit.)
應用
peptide synthesis
SMILES 字串
OC(=O)CNC(=O)OCc1ccccc1
InChI
1S/C10H11NO4/c12-9(13)6-11-10(14)15-7-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,11,14)(H,12,13)
InChI 密鑰
CJUMAFVKTCBCJK-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Z-Gly-OH又名N-苄氧羰基甘氨酸, 是一种氨基酸,广泛用于液相法多肽合成。
應用
Z-Gly-OH是多功能试剂,可合成各种化合物:
- 甘氨酸衍生肽,如Z-Gly-DL-Ala-OBzl和Z-Gly-L-Ala-OBzl
- 甘氨酸N-取代酰胺,如甘氨酸-N-甲酰胺盐酸和甘氨酸-N-异丙酰胺盐酸
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Studies on Separation of Amino Acids and Related Compounds. V. A Racemization Test in Peptide Synthesis by the Use of an Amino Acid Analyzer
Bulletin of the Chemical Society of Japan, 44, 3391-3395 (1971)
Duality of mechanism in the tetramethylfluoroformamidinium hexafluorophosphate-mediated synthesis of N-benzyloxycarbonylamino acid fluorides.
R Fiammengo et al.
The Journal of organic chemistry, 66(17), 5905-5910 (2001-08-21)
G K Scriba et al.
The Journal of pharmacy and pharmacology, 51(5), 549-553 (1999-07-20)
Glycine, which has weak anticonvulsant properties, has been shown to potentiate the activity of several antiepileptic drugs but not phenytoin. Recently, studies have shown that N-(benzyloxycarbonyl)glycine (Z-glycine) antagonized seizures more than glycine in addition to possessing activity in the maximal
D M Lambert et al.
Neuroreport, 5(7), 777-780 (1994-03-21)
Although glycine does not cross easily the blood-brain barrier, it exhibits at very high doses (10-40 mmol kg-1) a modest anticonvulsant activity. In this study, carbamate derivatives--N-benzyloxycarbonylglycine (Z-glycine) and N,tert-butoxycarbonylglycine (Boc-glycine)--have been compared with glycine. Z-glycine (1 mmol kg-1), but
David J Merkler et al.
Bioorganic & medicinal chemistry, 16(23), 10061-10074 (2008-10-28)
Peptidyl alpha-hydroxylating monooxygenase (PHM) functions in vivo towards the biosynthesis of alpha-amidated peptide hormones in mammals and insects. PHM is a potential target for the development of inhibitors as drugs for the treatment of human disease and as insecticides for
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務