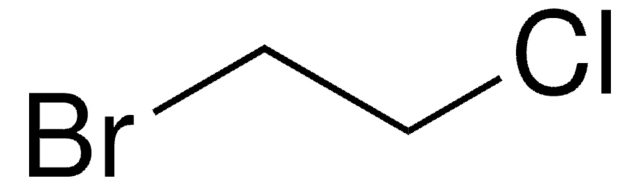
推薦產品
品質等級
化驗
99%
形狀
liquid
折射率
n20/D 1.4875 (lit.)
bp
80-82 °C/30 mmHg (lit.)
密度
1.488 g/mL at 25 °C (lit.)
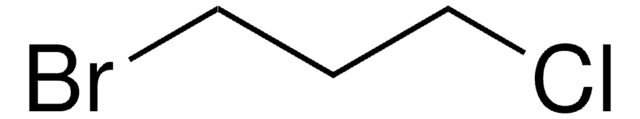
SMILES 字串
ClCCCCBr
InChI
1S/C4H8BrCl/c5-3-1-2-4-6/h1-4H2
InChI 密鑰
NIDSRGCVYOEDFW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
140.0 °F - closed cup
閃點(°C)
60 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
客戶也查看了
Stephen Verespy et al.
Scientific reports, 6, 24043-24043 (2016-04-08)
Allosteric partial inhibition of soluble, monomeric proteases can offer major regulatory advantages, but remains a concept on paper to date; although it has been routinely documented for receptors and oligomeric proteins. Thrombin, a key protease of the coagulation cascade, displays
Fulai Yang et al.
Scientific reports, 9(1), 18067-18067 (2019-12-04)
Camptothecin (CPT), a natural alkaloid isolated from Camptotheca acuminata Decne, is found to show potential insecticidal activities with unique action mechanisms by targeting at DNA-topoisomease I (Top1) complex and inducing cell apoptosis. To improve the efficacy against insect pests, two
Nan Li et al.
Toxicology in vitro : an international journal published in association with BIBRA, 41, 159-167 (2017-02-22)
The in vitro EpiSkin™ test method was validated in 2007 by the European Union Reference Laboratory for alternatives to animal testing (EURL ECVAM) as a full replacement method for the Draize acute skin irritation test and adopted in the OECD
Ngoc-Duc Doan et al.
Journal of peptide science : an official publication of the European Peptide Society, 21(5), 387-391 (2014-11-18)
The solid-phase synthesis of azapeptides possessing a C-terminal aza-residue has been accomplished by a protocol featuring regioselective alkylation of benzhydrylidene-aza-glycinamide and illustrated by the syntheses of [aza-Lys(6)] growth-hormone-releasing peptide-6 analogs.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務