About This Item
推薦產品
形狀
powder
品質等級
反應適用性
reagent type: oxidant
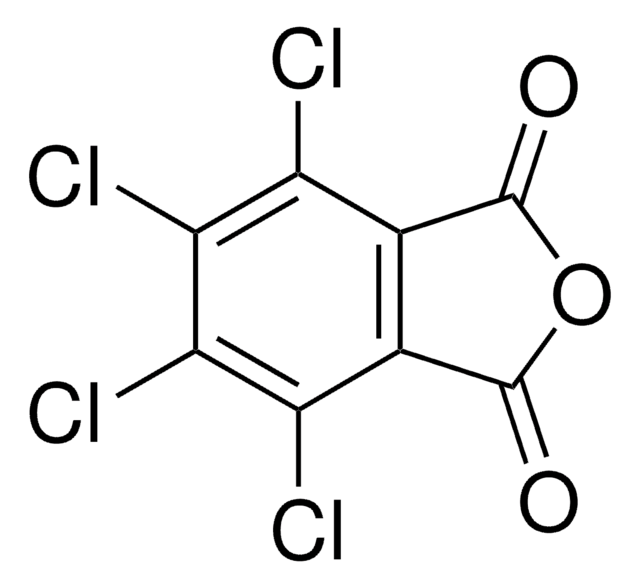
SMILES 字串
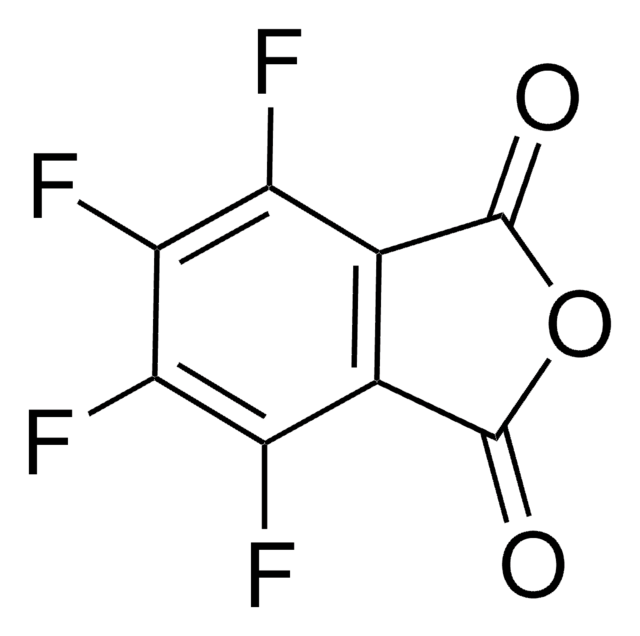
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
InChI 密鑰
UTRBHXSKVVPTLY-UHFFFAOYSA-N
一般說明
應用
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
文章
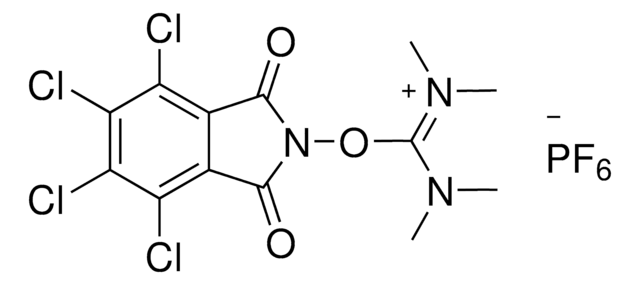
Professor Phil Baran and coworkers have developed a new reagent, N-Hydroxytetrachlorophthalimide (ALD00564), which provides a cheap, scalable, and safe synthetic alternative to highly used transformations like allylic oxidations, as well as Negishi and Suzuki–Miyaura type cross-coupling reactions.
Professor Phil Baran and coworkers have developed a new reagent, N-Hydroxytetrachlorophthalimide (ALD00564), which provides a cheap, scalable, and safe synthetic alternative to highly used transformations like allylic oxidations, as well as Negishi and Suzuki–Miyaura type cross-coupling reactions.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務