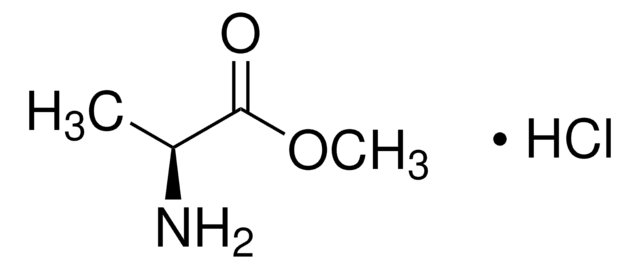
推薦產品
品質等級
化驗
≥99.0% (AT)
形狀
crystals
光學活性
[α]20/D −15.5±2°, c = 2% in H2O
反應適用性
reaction type: solution phase peptide synthesis
應用
peptide synthesis
SMILES 字串
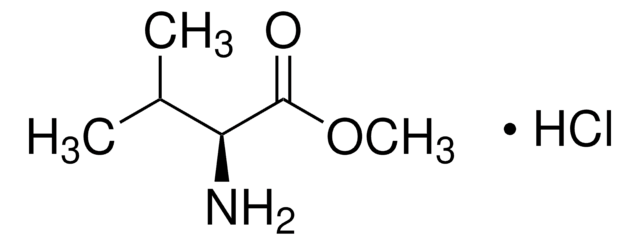
Cl.COC(=O)[C@H](N)C(C)C
InChI
1S/C6H13NO2.ClH/c1-4(2)5(7)6(8)9-3;/h4-5H,7H2,1-3H3;1H/t5-;/m1./s1
InChI 密鑰
KUGLDBMQKZTXPW-NUBCRITNSA-N
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Jan Labuta et al.
Nature communications, 4, 2188-2188 (2013-07-19)
Enantiomeric excess of chiral compounds is a key parameter that determines their activity or therapeutic action. The current paradigm for rapid measurement of enantiomeric excess using NMR is based on the formation of diastereomeric complexes between the chiral analyte and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務