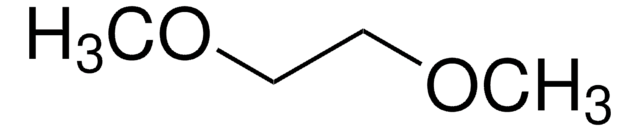
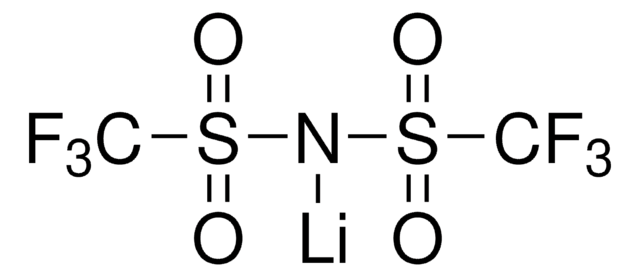
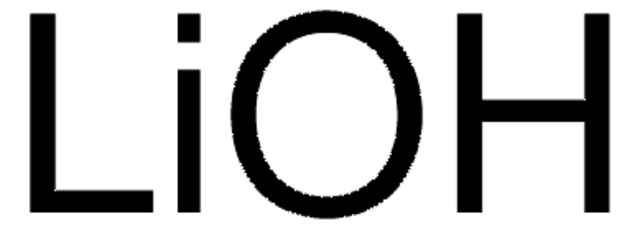
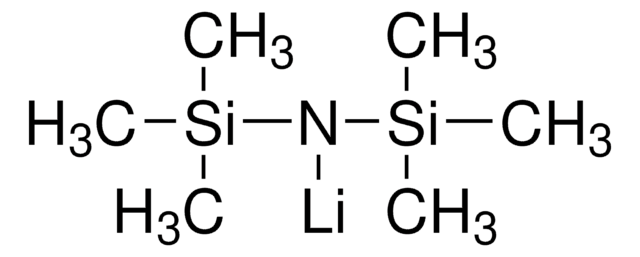推薦產品
等級
battery grade
品質等級
化驗
99.999% trace metals basis
形狀
powder
環保替代產品特色
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
雜質
≤15 ppm (trace metals analysis)
mp
264 °C (lit.)
溶解度
soluble (H2O: highly soluble(lit.); alcohols: soluble(lit.); acetone: soluble(lit.))
應用
battery manufacturing
SMILES 字串
[Li+].[O-][N+]([O-])=O
InChI
1S/Li.NO3/c;2-1(3)4/q+1;-1
InChI 密鑰
IIPYXGDZVMZOAP-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Lithium nitrate is a white, crystalline salt that is soluble in water, ethanol, methanol, pyridine, ammonia, and acetone. Importantly, it is also highly soluble up to 5 wt% in ether-based solvents such as dimethoxyethane (DME) and 1,3-dioxolane (DOL), but only soluble up to 1 wt% in carbonate-based solvents like ethylene carbonate (EC) and diethtyl carbonate (DEC).
Lithium nitrate is produced by reacting nitric acid and lithium carbonate, which evolves carbon dioxide and water. The resulting material is purified and dried.
Lithium nitrate is produced by reacting nitric acid and lithium carbonate, which evolves carbon dioxide and water. The resulting material is purified and dried.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
應用
Researchers and manufacturers use lithium nitrate in the synthesis of many lithium compounds. Our 99.999% lithium nitrate is well-suited as a reagent for solid-state syntheses of lithium metal oxides, especially where purity is of high importance, for example, when making products whose fundamental properties are under investigation.
Our 99.999% lithium nitrate is also well-suited for use as an additive to electrolytes in lithium-sulfur batteries and lithium metal batteries. Lithium nitrate can passivate the surface of lithium metal and suppress the redox shuttle of the dissolved lithium polysulfides on the lithium anode. In one study, the addition of 0.3 M LiNO3 nearly doubled the gravimetric capacity of lithium-sulfide batteries. Another study found that the dissolution of 1 to 5 wt% LiNO3 to the electrolyte suppressed growth of lithium dendrites and extended cycle lifetimes. Similarly beneficial effects of lithium nitrate as an additive have been observed with Li2S cathodes, carbon nanofiber-encapsulated sulfur cathodes, cobalt sulfide (Co3S4) cathodes, and polyacrylonitrile-sulfur composite cathodes. Even lithium metal anodes with LiNi0.8Co0.15Al0.05O2 (NCA) cathodes with LiNO3 added to the electrolyte showed higher coulombic efficiencies and suppressed dendrite formation compared to the electrolyte without LiNO3.
Our 99.999% lithium nitrate is also well-suited for use as an additive to electrolytes in lithium-sulfur batteries and lithium metal batteries. Lithium nitrate can passivate the surface of lithium metal and suppress the redox shuttle of the dissolved lithium polysulfides on the lithium anode. In one study, the addition of 0.3 M LiNO3 nearly doubled the gravimetric capacity of lithium-sulfide batteries. Another study found that the dissolution of 1 to 5 wt% LiNO3 to the electrolyte suppressed growth of lithium dendrites and extended cycle lifetimes. Similarly beneficial effects of lithium nitrate as an additive have been observed with Li2S cathodes, carbon nanofiber-encapsulated sulfur cathodes, cobalt sulfide (Co3S4) cathodes, and polyacrylonitrile-sulfur composite cathodes. Even lithium metal anodes with LiNi0.8Co0.15Al0.05O2 (NCA) cathodes with LiNO3 added to the electrolyte showed higher coulombic efficiencies and suppressed dendrite formation compared to the electrolyte without LiNO3.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 3
儲存類別代碼
5.1B - Oxidizing hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Yuan Yang et al.
Journal of the American Chemical Society, 134(37), 15387-15394 (2012-08-23)
Li(2)S is a high-capacity cathode material for lithium metal-free rechargeable batteries. It has a theoretical capacity of 1166 mAh/g, which is nearly 1 order of magnitude higher than traditional metal oxides/phosphates cathodes. However, Li(2)S is usually considered to be electrochemically
Role of LiNO3 in rechargeable lithium/sulfur battery.
Zhang S, et al.
Electrochimica Acta, 70, 344-348 (2012)
The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth.
Weiyang Li et al.
Nature communications, 6, 7436-7436 (2015-06-18)
Lithium metal has shown great promise as an anode material for high-energy storage systems, owing to its high theoretical specific capacity and low negative electrochemical potential. Unfortunately, uncontrolled dendritic and mossy lithium growth, as well as electrolyte decomposition inherent in
Chong Yan et al.
Angewandte Chemie (International ed. in English), 57(43), 14055-14059 (2018-08-11)
The lithium metal anode is regarded as a promising candidate in next-generation energy storage devices. Lithium nitrate (LiNO3 ) is widely applied as an effective additive in ether electrolyte to increase the interfacial stability in batteries containing lithium metal anodes.
On the Surface Chemical Aspects of Very High Energy Density, Rechargeable Li?Sulfur Batteries.
Aurbach D, et al.
Journal of the Electrochemical Society, 156, A694-A694 (2009)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務