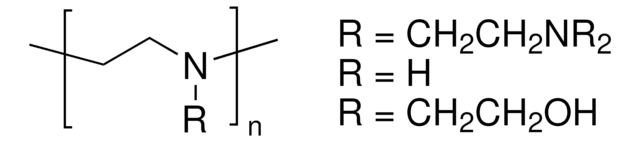推薦產品
品質等級
化驗
≥95%
形狀
powder or crystals
反應適用性
reagent type: catalyst
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
環保替代類別
, Aligned
儲存溫度
−20°C
SMILES 字串
[H]C1([H])C([H])([H])O[C@@](C([H])([H])O[Si](C([H])([H])[H])(C([H])([H])[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])([H])[C@](O[H])([H])[C@]1([H])O[H]
InChI
1S/C12H26O4Si/c1-12(2,3)17(4,5)16-8-10-11(14)9(13)6-7-15-10/h9-11,13-14H,6-8H2,1-5H3/t9-,10-,11+/m1/s1
InChI 密鑰
KKFGQPZYDWCKRC-MXWKQRLJSA-N
相關類別
一般說明
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
應用
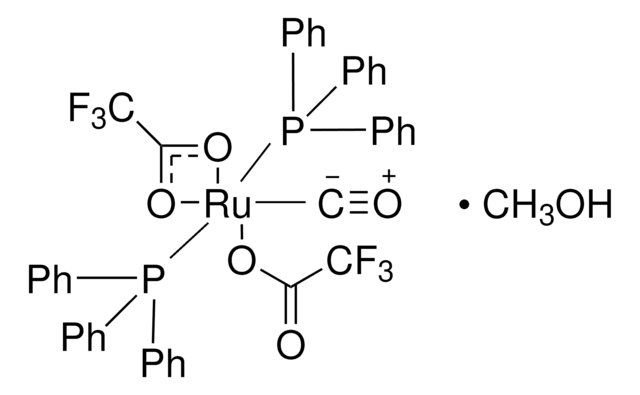
6-Tertbutyldimethylsilyl-1,2-dihydroglucal (TBS-DHG) was reported by the Morken Lab to be an effective carbohydrate-derived catalyst for enantioselective diboration of alkenes. Related capabilities were observed with the dihydrorhamnal (DHR) catalyst (901237). TBS-DHG has also been used in ruthenium(0)catalyzed transfer hydrogenation cycloadditions.
其他說明
- Carbohydrate-Catalyzed Enantioselective Alkene Diboration:Enhanced Reactivity of 1,2-Bonded Diboron Complexes
- Diols, α-Ketols, and Diones as 22π Components in [2+2+2] Cycloadditions of 1,6-Diynes via Ruthenium(0)-Catalyzed Transfer Hydrogenation
- Carbohydrate/DBU Cocatalyzed Alkene Diboration: Mechanistic Insight Provides Enhanced Catalytic Efficiency and Substrate Scope
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Zachary A Kasun et al.
Chemical communications (Cambridge, England), 50(56), 7545-7547 (2014-06-04)
A new method for the ring expansion of cyclic diols is described. Using improved conditions for the ruthenium(0) catalyzed cycloaddition of cyclic 1,2-diols with 1,3-dienes, fused [n.4.0] bicycles (n = 3-6) are formed, which upon exposure to iodosobenzene diacetate engage
Hiroki Sato et al.
Journal of the American Chemical Society, 138(50), 16244-16247 (2016-12-10)
The first use of vicinal diols, ketols, or diones as 2
Lichao Fang et al.
Journal of the American Chemical Society, 138(8), 2508-2511 (2016-02-09)
Catalytic enantioselective diboration of alkenes is accomplished with readily available carbohydrate-derived catalysts. Mechanistic experiments suggest the intermediacy of 1,2-bonded diboronates.
Lu Yan et al.
Journal of the American Chemical Society, 140(10), 3663-3673 (2018-02-15)
A mechanistic investigation of the carbohydrate/DBU cocatalyzed enantioselective diboration of alkenes is presented. These studies provide an understanding of the origin of stereoselectivity and also reveal a strategy for enhancing reactivity and broadening the substrate scope.
文章
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![Dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/374/597/f7932c5b-0448-498b-8254-f8ce1b9a4612/640/f7932c5b-0448-498b-8254-f8ce1b9a4612.png)