推薦產品
品質等級
產品線
ReagentPlus®
化驗
99%
形狀
powder
光學活性
[α]22/D +40.0°, c = 1 in methanol
反應適用性
reaction type: C-H Activation
reaction type: solution phase peptide synthesis
reagent type: ligand
reaction type: Peptide Synthesis
mp
171-173 °C (lit.)
應用
peptide synthesis
官能基
amine
carboxylic acid
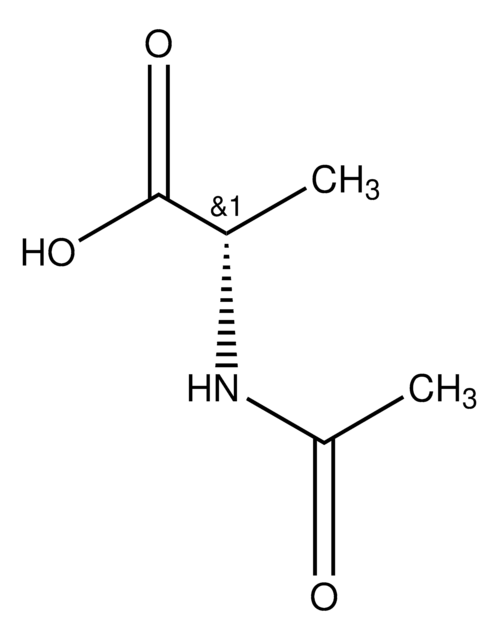
SMILES 字串
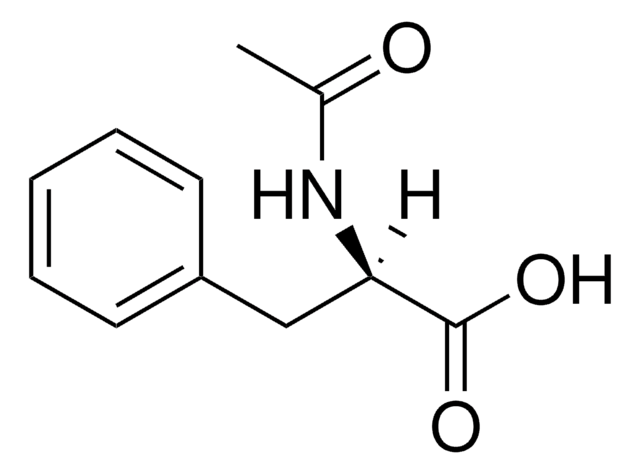
CC(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI
1S/C11H13NO3/c1-8(13)12-10(11(14)15)7-9-5-3-2-4-6-9/h2-6,10H,7H2,1H3,(H,12,13)(H,14,15)/t10-/m0/s1
InChI 密鑰
CBQJSKKFNMDLON-JTQLQIEISA-N
尋找類似的產品? 前往 產品比較指南
一般說明
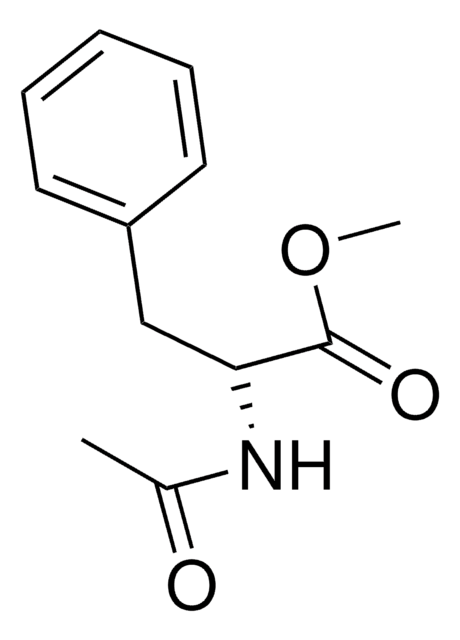
N-乙酰基-L-苯基丙氨酸是L-苯基丙氨酸的乙酰基类似物。它被广泛用作反应物,以合成N-乙酰基-L-苯基丙氨酸的甲基或乙基酯,可作为肽合成中的通用构件。
應用
N-乙酰基-L-苯基丙氨酸可用作合成以下物质的反应物:
- 使用Mukaiyama试剂通过甲醇酯化反应产生N-乙酰苯丙氨酸甲酯。
- 通过铑催化氢化反应产生乙酰氨基环己烷丙酸。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Surfactant-protease complex as a novel biocatalyst for peptide synthesis in hydrophilic organic solvents
Okazaki S, et al.
Enzyme and Microbial Technology, 26(2-4), 159-164 (2000)
Corinna Neuber et al.
Analytical chemistry, 86(18), 9065-9073 (2014-08-20)
Sphingosine 1-phosphate (S1P), a bioactive lipid involved in various physiological processes, can be irreversibly degraded by the membrane-bound S1P lyase (S1PL) yielding (2E)-hexadecenal and phosphoethanolamine. It is discussed that (2E)-hexadecenal is further oxidized to (2E)-hexadecenoic acid by the long-chain fatty
K Y Xu
Biochemistry, 28(17), 6894-6899 (1989-08-22)
A combination of competitive labeling with [3H]acetic anhydride [Kaplan, H., Stevenson, K. J., & Hartley, B. S. (1971) Biochem. J. 124, 289-299] and immunoaffinity chromatography is described that permits the assignment of the acid dissociation constant and the absolute nucleophilicity
E Jellum et al.
Scandinavian journal of clinical and laboratory investigation. Supplementum, 184, 21-26 (1986-01-01)
Urinary organic acid profiles of patients with Maple Syrup Urine Disease (MSUD), hereditary tyrosinemia and phenylketonuria (PKU) have been studied by means of capillary GC-MS-computer technique. In addition to the characteristic metabolites of these disorders, increased amounts of N-acetylleucine, N-acetylisoleucine
Edward A Lemke
Methods in molecular biology (Clifton, N.J.), 751, 3-15 (2011-06-16)
Studies of protein structure and function using single-molecule fluorescence resonance energy transfer (smFRET) benefit dramatically from the ability to site-specifically label proteins with small fluorescent dyes. Genetically encoding the unnatural amino acid (UAA) p-acetylphenylalanine is an efficient way to introduce
Chromatograms
application for HPLC我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務