全部照片(2)
About This Item
線性公式:
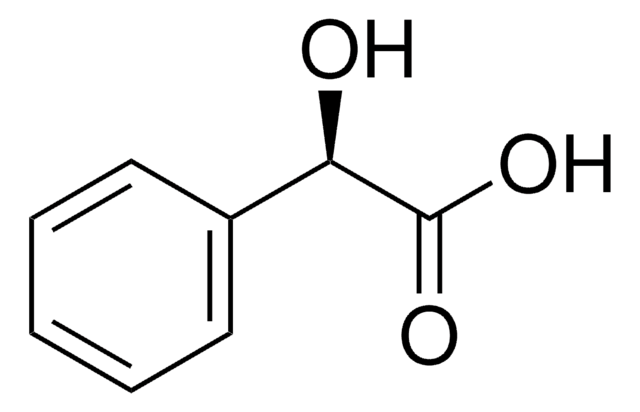
C6H5CH(OH)CO2H
CAS號碼:
分子量::
152.15
Beilstein:
2208678
EC號碼:
MDL號碼:
分類程式碼代碼:
12352002
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
產品線
ReagentPlus®
化驗
≥99%
形狀
crystals
mp
131-134 °C (lit.)
132-138 °C
官能基
carboxylic acid
hydroxyl
phenyl
SMILES 字串
O[C@H](C(O)=O)c1ccccc1
InChI
1S/C8H8O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7,9H,(H,10,11)/t7-/m0/s1
InChI 密鑰
IWYDHOAUDWTVEP-ZETCQYMHSA-N
尋找類似的產品? 前往 產品比較指南
應用
(S)-(+)-Mandelic acid can be used as a starting material to synthesize (S)-cyclohexenyl phenyl glycoxilic acid, an optically active tertiary α-hydroxy acid component of (S)-oxybutynin.
It is a versatile reagent used in the resolution of racemates and the preparation of amides.
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
>374.0 °F
閃點(°C)
> 190 °C
The Journal of Organic Chemistry, 58, 2313-2313 (1993)
Tetrahedron, 50, 5049-5049 (1994)
Chiral mandelic acid template provides a highly practical solution for (S)-oxybutynin synthesis.
Grover P T, et al.
The Journal of Organic Chemistry, 65(19), 6283-6287 (2000)
Mara Reifenrath et al.
Metabolic engineering communications, 7, e00079-e00079 (2018-10-30)
Mandelic acid is an important aromatic fine chemical and is currently mainly produced via chemical synthesis. Recently, mandelic acid production was achieved by microbial fermentations using engineered Escherichia coli and Saccharomyces cerevisiae expressing heterologous hydroxymandelate synthases (hmaS). The best-performing strains
Synthetic Communications, 23, 2761-2761 (1993)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務