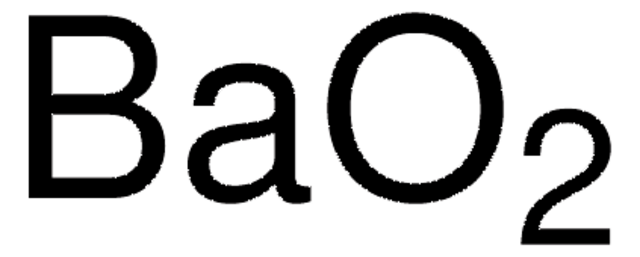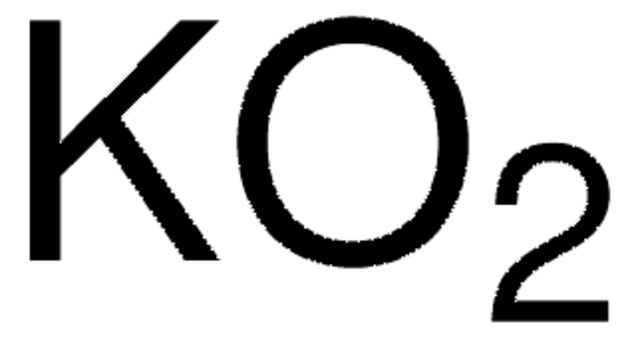推薦產品
等級
anhydrous
品質等級
化驗
≥86.0% (RT)
形狀
powder
損耗
≤0.1% loss on drying
負離子痕跡
chloride (Cl-): ≤0.05%
正離子痕跡
Fe: ≤0.02%
heavy metals (as Pb): ≤0.01%
SMILES 字串
[Ba++].[O-][O-]
InChI
1S/Ba.O2/c;1-2/q+2;-2
InChI 密鑰
ZJRXSAYFZMGQFP-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
Barium peroxide is a precursor material used for synthesis of novel magnetic and superconducting oxides. It also finds application in thermochemical energy storage systems.
分析報告
Substances insoluble in dilute hydrochlorid acid ≤ 1.5 %
Substances not precipitated by diluted sulfuric acid (as sulphates) ≤ 2.0 %
Substances not precipitated by diluted sulfuric acid (as sulphates) ≤ 2.0 %
訊號詞
Danger
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1B
儲存類別代碼
5.1A - Strongly oxidizing hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Honda, R., et al.
Physica C, 426, 613-617 (2005)
A J Carrillo et al.
Physical chemistry chemical physics : PCCP, 18(11), 8039-8048 (2016-03-01)
The barium peroxide-based redox cycle was proposed in the late 1970s as a thermochemical energy storage system. Since then, very little attention has been paid to such redox couples. In this paper, we have revisited the use of reduction-oxidation reactions
Ba2IrO4: A spin-orbit Mott insulating quasi-two-dimensional antiferromagnet
Okabe, H., et al.
Physical Review. B, Condensed Matter and Materials Physics, 83, 155118-155118 (2011)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務