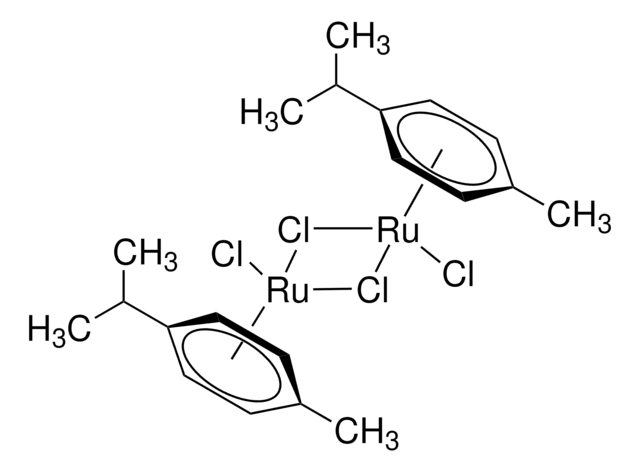
推薦產品
形狀
solid
品質等級
光學活性
[α]22/D +339°, c = 0.5% in chloroform
反應適用性
core: ruthenium
reagent type: catalyst
mp
260-268 °C
儲存溫度
2-8°C
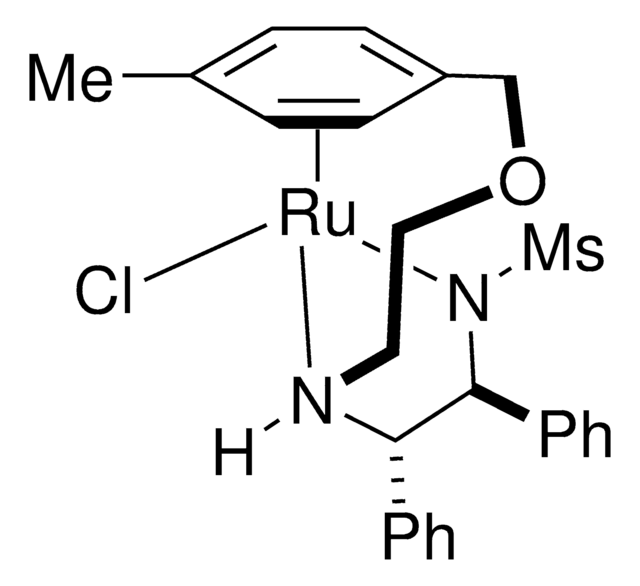
SMILES 字串
Cc1ccc(cc1)S(=O)(=O)N2[C@H]([C@@H]([N@@H](CCCc3ccccc3)[Ru]2Cl)c4ccccc4)c5ccccc5
InChI
1S/C30H31N2O2S.ClH.Ru/c1-24-19-21-28(22-20-24)35(33,34)32-30(27-17-9-4-10-18-27)29(26-15-7-3-8-16-26)31-23-11-14-25-12-5-2-6-13-25;;/h2-10,12-13,15-22,29-31H,11,14,23H2,1H3;1H;/q-1;;+2/p-1/t29-,30-;;/m0../s1
InChI 密鑰
MDABGVLQRDDWLY-ARDORAJISA-M
相關類別
應用
[(S,S)-Teth-TsDpen RuCl] is a catalyst for the asymmetric hydrogenation of ketones. It can be used as a catalyst:
- In the reduction of a ketone intermediate for the synthesis of esketamine.
- In asymmetric hydrogenation of acetylenic ketones.
- For the preparation of (3S,6S)-1,8-diphenylocta-1,7-diyne-3,6-diol starting from 1,8-diphenylocta-1,7-diyne-3,6-dione.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Iron cyclopentadienone complexes derived from C 2-symmetric bis-propargylic alcohols; preparation and applications to catalysis
Hodgkinson R, et al.
Dalton Transactions, 45(9), 3992-4005 (2016)
Aidan M Hayes et al.
Journal of the American Chemical Society, 127(20), 7318-7319 (2005-05-19)
Ruthenium dimer 6 (readily available in two steps from TsDPEN) is converted directly to monomeric asymmetric transfer hydrogenation catalyst 3 in situ under the conditions employed for ketone reduction. Catalyst 3 is a significantly more active catalyst for this application
Asymmetric transfer hydrogenation of functionalized acetylenic ketones
Fang Z and Wills M
The Journal of Organic Chemistry, 78(17), 8594-8605 (2013)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![RuCl(p-异丙基甲苯)[(S,S)-Ts-DPEN]](/deepweb/assets/sigmaaldrich/product/structures/596/849/f8e3d2d8-a02e-430e-b0ed-c208cea3a6fb/640/f8e3d2d8-a02e-430e-b0ed-c208cea3a6fb.png)
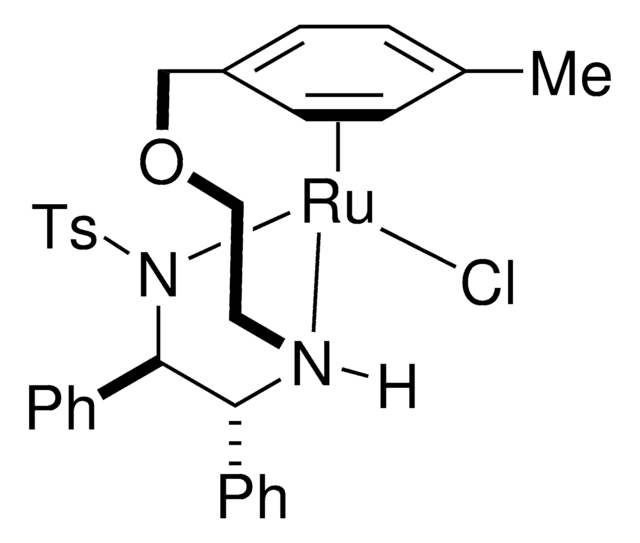
![[(R,R)-Teth-TsDpen RuCl]](/deepweb/assets/sigmaaldrich/product/structures/307/962/59412599-3794-437d-8a39-b88a036b7ca1/640/59412599-3794-437d-8a39-b88a036b7ca1.png)
 95%](/deepweb/assets/sigmaaldrich/product/structures/151/609/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1/640/eeb99dc1-9ef2-49d8-b255-6b5e2519fee1.png)
![RuCl2[(R)-DM-BINAP][(R,R)-DPEN]](/deepweb/assets/sigmaaldrich/product/structures/425/564/80b6a39d-c641-4583-8b52-ab506b343228/640/80b6a39d-c641-4583-8b52-ab506b343228.png)
![RuCl2[(R)-DM-BINAP][(R)-DAIPEN]](/deepweb/assets/sigmaaldrich/product/structures/355/628/d82e5e5d-23fd-468a-87ca-db4c45677898/640/d82e5e5d-23fd-468a-87ca-db4c45677898.png)
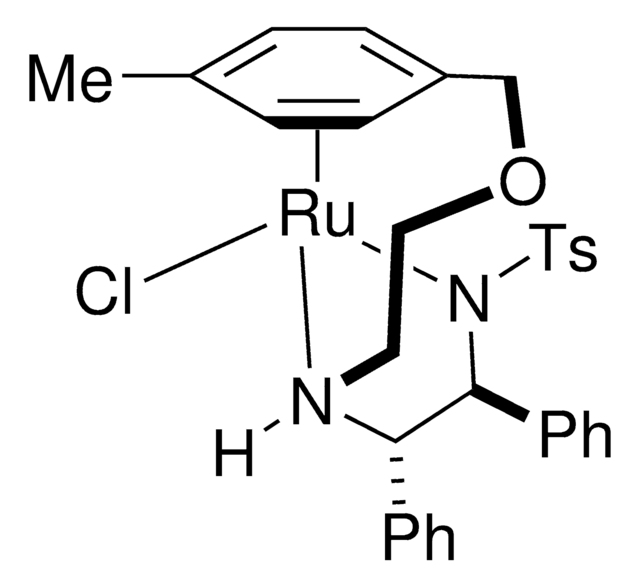
![RuCl(p-异丙基甲苯)[(R,R)-Ts-DPEN]](/deepweb/assets/sigmaaldrich/product/structures/161/670/212e6eb8-dce1-4397-a5ef-3e2e3a296e8c/640/212e6eb8-dce1-4397-a5ef-3e2e3a296e8c.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化镍(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)