全部照片(3)
About This Item
經驗公式(希爾表示法):
C8H5N3
CAS號碼:
分子量::
143.15
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
96%
形狀
solid
反應適用性
reaction type: C-C Bond Formation
mp
98-106 °C
SMILES 字串
N#Cn1cnc2ccccc12
InChI
1S/C8H5N3/c9-5-11-6-10-7-3-1-2-4-8(7)11/h1-4,6H
InChI 密鑰
SGCJHVTWXFWDOD-UHFFFAOYSA-N
應用
1-Cyanobenzimidazole can be used:
- To prepare alkyl benzimidazole-1-carboximidates and 1H-benzimidazole-1-carbohydrazonamide by reacting with aliphatic alcohols and excess of hydrazine, respectively.
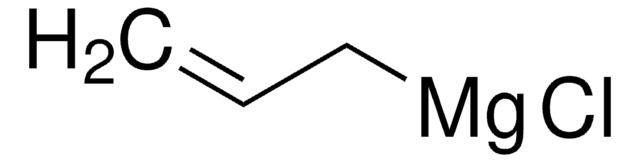
- As an electrophilic cyanating reagent in cyanation reactions including aryl and heteroaryl Grignard reagents.
Reacant for:
- Electrophilic aromatic substitution reactions
- Electrophilic cyanation of aryl and heteroaryl Grignard reagents
- Hydrolysis in alkalyne solutions
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
New strategies and applications using electrophilic cyanide-transfer reagents under transition metal-free conditions
Schorgenhumer J and Waser M
Organic Chemistry Frontiers : An International Journal of Organic Chemistry / Royal Society of Chemistry, 3(11), 1535-1540 (2016)
1H-Benzimidazole-1-carbohydrazonamide
Sokolov AV, et al.
Acta Crystallographica Section E, Structure Reports Online, 62(8), o3209-o3210 (2006)
Transnitrilation from dimethylmalononitrile to aryl grignard and lithium reagents: A practical method for aryl nitrile synthesis
Reeves JT, et al.
Journal of the American Chemical Society, 137(29), 9481-9488 (2015)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務