全部照片(1)
About This Item
經驗公式(希爾表示法):
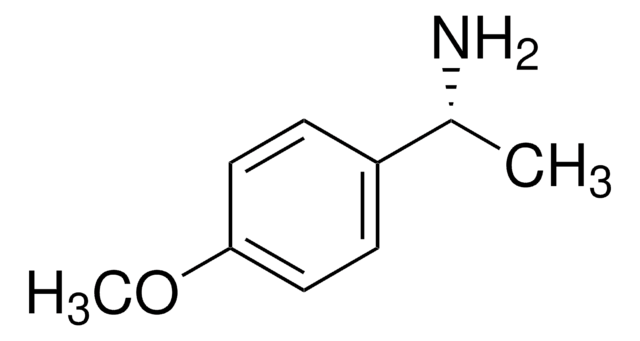
C9H13NO
CAS號碼:
分子量::
151.21
Beilstein:
3196456
MDL號碼:
分類程式碼代碼:
12352112
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
≥98.5% (GC)
99%
形狀
liquid
光學純度
enantiomeric excess: ≥98.5%
密度
1.024 g/mL at 20 °C (lit.)
官能基
amine
SMILES 字串
COc1ccc(cc1)[C@H](C)N
InChI
1S/C9H13NO/c1-7(10)8-3-5-9(11-2)6-4-8/h3-7H,10H2,1-2H3/t7-/m0/s1
InChI 密鑰
JTDGKQNNPKXKII-ZETCQYMHSA-N
尋找類似的產品? 前往 產品比較指南
應用
(S)-(−)-4-Methoxy-α-methylbenzylamine is employed in the synthesis of S(+)-4-(1-phenylethylamino)quinazolines, as human immunoglobuline E inhibitor and haloaryl-β-amino acids. It is also used as a precursor to prepare chiral intermediate in the total synthesis of solanoeclepin A.
法律資訊
ChiPros is a registered trademark of BASF SE
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
S (+)-4-(1-phenylethylamino) quinazolines as inhibitors of human immunoglobuline E synthesis: potency is dictated by stereochemistry and atomic point charges at N-1.
Berger M, et al.
Journal of Medicinal Chemistry, 44(18), 3031-3038 (2001)
The asymmetric synthesis of β-haloaryl-β-amino acid derivatives.
Bull S D, et al.
Synlett, 2000(09), 1257-1260 (2000)
Novel Synthesis of the ABC Rings of Solanoeclepin A.
Lin Y T, et al.
Organic Letters, 16(22), 5948-5951 (2014)
文章
ChiPros Chiral Amines
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務