推薦產品
化驗
97%
形狀
solid
mp
74-78 °C
官能基
amine
oxime
phenyl
SMILES 字串
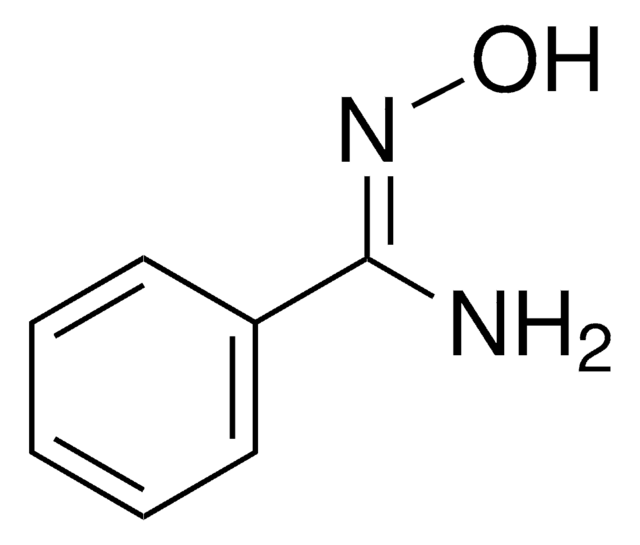
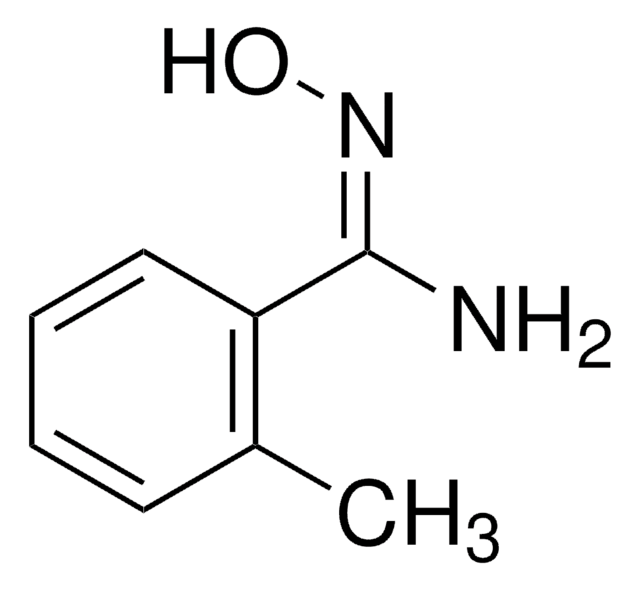
N\C(=N\O)c1ccccc1
InChI
1S/C7H8N2O/c8-7(9-10)6-4-2-1-3-5-6/h1-5,10H,(H2,8,9)
InChI 密鑰
MXOQNVMDKHLYCZ-UHFFFAOYSA-N
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
B Clement et al.
Archiv der Pharmazie, 322(7), 431-435 (1989-07-01)
At pH 7.4 neither benzamidine (1) is ring-hydroxylated nor benzamidoxime (2) is N-hydroxylated, reduced or ring-hydroxylated by aerobic incubations with microsomal fractions (12000 g supernatant, microsomes) of rabbit liver homogenates and NADPH. Products of hydrolytic processes are also not detected.
B Clement et al.
Journal of cancer research and clinical oncology, 114(4), 363-368 (1988-01-01)
The genotoxic potentials of benzamidine and benzamidoxime were determined to study the toxicological relevance of the metabolic N-oxygenation (N-hydroxylation) of benzamidines to benzamidoximes. Benzamidoxime induced DNA single-strand breaks (in rat hepatocytes) and DNA amplification in SV40-transformed hamster cells. In the
R Reh et al.
Xenobiotica; the fate of foreign compounds in biological systems, 38(9), 1177-1190 (2008-07-09)
1. This study investigates the enzymatic reduction of N-hydroxylated amidines by porcine adipose tissue and the possible involvement of stearoyl-CoA desaturase (SCD). 2. The reduction of the model substrate benzamidoxime was studied with porcine adipose tissue microsomes and partially purified
Khajadpai Thipyapong et al.
Inorganic chemistry, 50(3), 992-998 (2011-01-14)
In search of benzamidoxime (BHam) derivatives that provide a single (99m)Tc-labeled compound of high in vivo stability, we synthesized three N-alkyl compounds of benzamidoxime (BHam) ligand. They provided a single (99m)Tc-labeled compound by ligand exchange reaction of (99m)Tc-glucoheptonate in high
A Jousserandot et al.
Biochemistry, 37(49), 17179-17191 (1998-12-23)
Oxidation by rat liver microsomes of 13 compounds involving a C=N(OH) function (including N-hydroxyguanidines, amidoximes, ketoximes, and aldoximes) was found to occur with the release of nitrogen oxides such as NO, NO2-, and NO3-. The greatest activities were observed with
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務