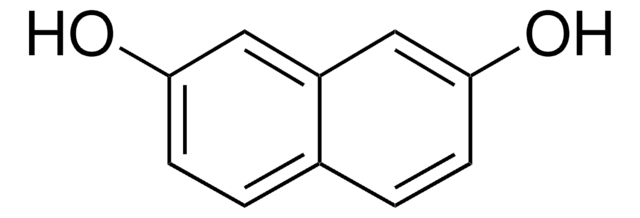
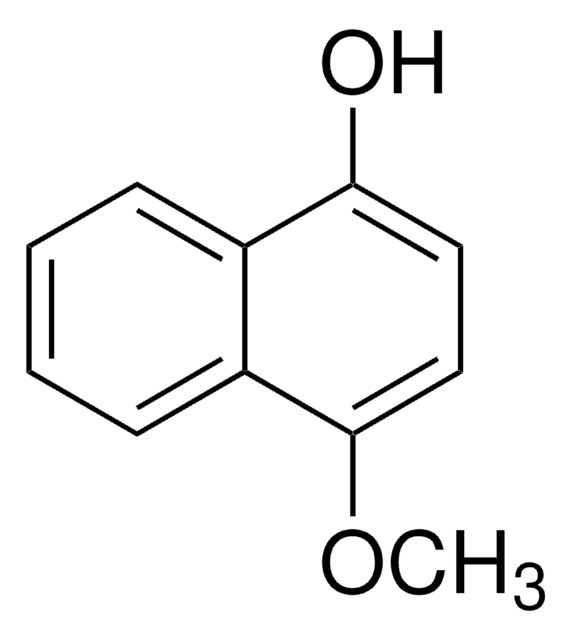
推薦產品
等級
technical
品質等級
化驗
≥90% (HPLC)
形狀
crystals
mp
191-192 °C (dec.)
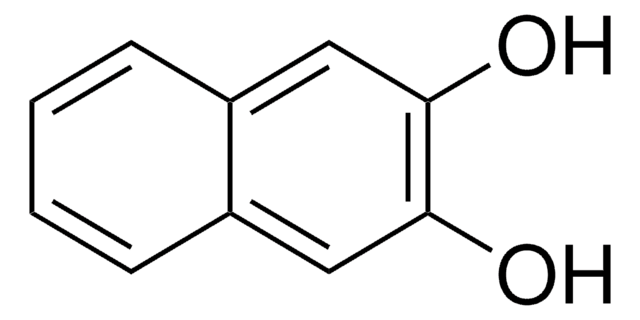
SMILES 字串
Oc1ccc(O)c2ccccc12
InChI
1S/C10H8O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6,11-12H
InChI 密鑰
PCILLCXFKWDRMK-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Autoxidation of naphthohydroquinones: effects of metals, chelating agents, and superoxide dismutase.
R Munday
Free radical biology & medicine, 22(4), 689-695 (1997-01-01)
At neutral pH, 1,4-naphthohydroquinone and 2-methyl-1,4-naphthohydroquinone readily autoxidize to the corresponding quinones. In an unpurified phosphate buffer, the autoxidation of both substances proceeded in a linear fashion after a brief lag phase. Addition of a chelating agent or purification of
R Munday
Free radical research, 32(3), 245-253 (2000-03-24)
The rates of autoxidation of a number of pure naphthohydroquinones have been determined, and the effects of pH, superoxide dismutase (SOD) and of the parent naphthoquinone on the oxidation rates have been investigated. Most compounds were slowly oxidised in acid
Selective nonpeptidic inhibitors of herpes simplex virus type 1 and human cytomegalovirus proteases.
M Matsumoto et al.
Biological & pharmaceutical bulletin, 24(3), 236-241 (2001-03-21)
The proteases encoded by herpesviruses including herpes simplex virus type 1 (HSV-1) and human cytomegalovirus (HCMV) are attractive targets for antiviral drug development because of their important roles in viral replication. We randomly screened a chemical compound library for inhibitory
María Teresa Molina et al.
The Journal of organic chemistry, 74(24), 9573-9575 (2009-11-27)
The NHC-catalyzed conjugate hydroacylation of 1,4-naphthoquinones allows for the synthesis of monoacylated 1,4-dihydroxynaphthalene derivatives. These targets, difficult to prepare selectively by standard protocols, represent important intermediates in the elaboration of highly substituted 1,4-naphthoquinone derivatives, which constitute relevant pharmaceutical scaffolds. High
T Ishii et al.
Free radical biology & medicine, 8(1), 21-24 (1990-01-01)
The autoxidation of 1,4-naphthohydroquinone, in a phosphate, EDTA buffer at pH 7.4, exhibits an autocatalysis whose lag phase becomes more pronounced in the presence of either the Cu,Zn- or the Mn-containing superoxide dismutases. In contrast, the autoxidation of a second
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務