推薦產品
品質等級
化驗
≥89.0%
90%
形狀
lumps
官能基
amine
fluoro
thiourea
SMILES 字串
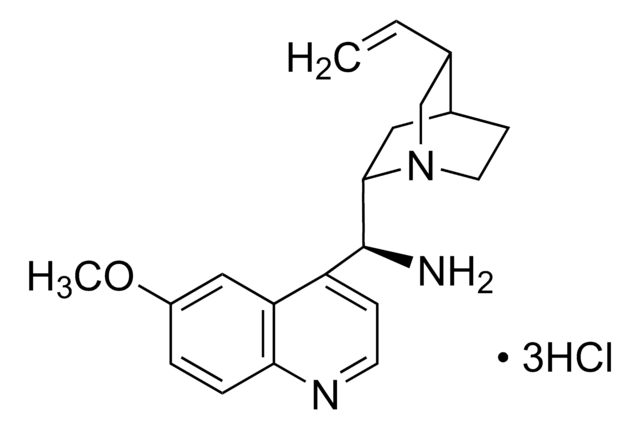
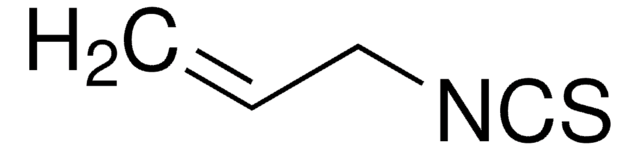
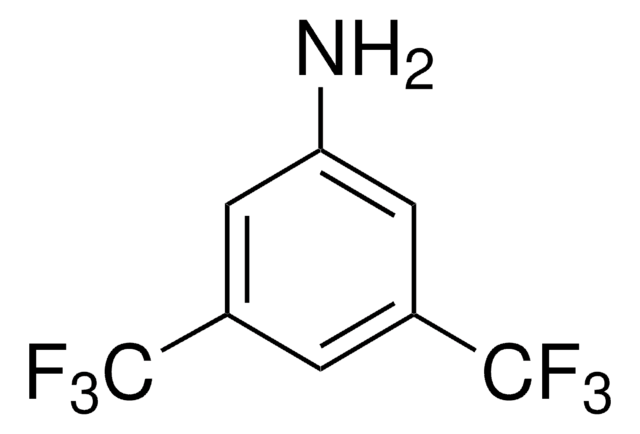
COc1ccc2nccc([C@H](NC(=S)Nc3cc(cc(c3)C(F)(F)F)C(F)(F)F)C4CC5CCN4C[C@@H]5C=C)c2c1
InChI
1S/C29H28F6N4OS/c1-3-16-15-39-9-7-17(16)10-25(39)26(22-6-8-36-24-5-4-21(40-2)14-23(22)24)38-27(41)37-20-12-18(28(30,31)32)11-19(13-20)29(33,34)35/h3-6,8,11-14,16-17,25-26H,1,7,9-10,15H2,2H3,(H2,37,38,41)/t16-,17-,25-,26-/m0/s1
InChI 密鑰
IQMKPBFOEWWDIQ-FRSFCCSCSA-N
應用
N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8a,9S)-6′-methoxy-9-cinchonanyl]thiourea is a bifunctional cinchona organocatalyst, which can be used to synthesize:
- Stereoselective diaryl(nitro)butanone via enantioselective Michael addition of nitromethane to chalcones.
- Enantioselective β-amino acids via asymmetric Mannich reaction of malonates with aryl and alkyl imines.
- The synthesis of 3-indolylmethanamines by the reaction of indoles with imines via asymmetric Friedel-Crafts reaction.
- The enantioselective conjugate addition of active methylene compounds to enones to obtain the corresponding addition products.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
客戶也查看了
The Mannich reaction of malonates with simple imines catalyzed by bifunctional cinchona alkaloids: enantioselective synthesis of ?-amino acids
Song J, et al.
Journal of the American Chemical Society, 128(18), 6048-6049 (2006)
Asymmetric Friedel- Crafts reaction of indoles with imines by an organic catalyst
Wang Y-Q, et al.
Journal of the American Chemical Society, 128(25), 8156-8157 (2006)
Urea- and thiourea-substituted cinchona alkaloid derivatives as highly efficient bifunctional organocatalysts for the asymmetric addition of malonate to nitroalkenes: inversion of configuration at C9 dramatically improves catalyst performance.
Séamus H McCooey et al.
Angewandte Chemie (International ed. in English), 44(39), 6367-6370 (2005-09-02)
Benedek Vakulya et al.
Organic letters, 7(10), 1967-1969 (2005-05-07)
Cinchona alkaloid-derived chiral bifunctional thiourea organocatalysts were synthesized and applied in the Michael addition between nitromethane and chalcones with high ee and chemical yields.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)
![N-[(1R,2R)-2-(1-哌啶基)环己基]-N′-[4-(三氟甲基)苯基]四酰胺 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)
![N-[3,5-双(三氟甲基)苯基]-N′-[(8a,9S)-10,11-二氢-6′-甲氧基-9-金鸡宁]硫脲 90%](/deepweb/assets/sigmaaldrich/product/structures/321/413/b3c0b73b-9ee7-4dea-b9cd-795beb4cdbfa/640/b3c0b73b-9ee7-4dea-b9cd-795beb4cdbfa.png)
![1-[3,5-Bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(−)-2-(dimethylamino)cyclohexyl]thiourea](/deepweb/assets/sigmaaldrich/product/structures/384/772/d336462c-f438-446d-be0c-4064705213cc/640/d336462c-f438-446d-be0c-4064705213cc.png)
![1,3-双[3,5-双(三氟甲基)苯基]硫脲 95%](/deepweb/assets/sigmaaldrich/product/structures/191/427/0218c99c-65b9-4963-938c-c47a5790dfc5/640/0218c99c-65b9-4963-938c-c47a5790dfc5.png)
![(S)-2-[[3,5-双(三氟甲基)苯基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)