全部照片(4)
About This Item
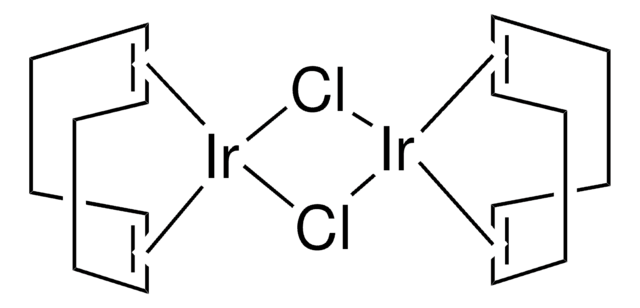
線性公式:
[Ir(OCH3)(C8H12)]2
CAS號碼:
分子量::
662.86
MDL號碼:
分類程式碼代碼:
12161600
PubChem物質ID:
NACRES:
NA.22
推薦產品
形狀
crystals
品質等級
反應適用性
core: iridium
reagent type: catalyst
reaction type: C-H Activation
mp
154-179 °C (D)
儲存溫度
−20°C
SMILES 字串
C[O+]1[Ir-]2[O+](C)[Ir-]12.C3CC=CCCC=C3.C4CC=CCCC=C4
InChI
1S/2C8H12.2CH3O.2Ir/c2*1-2-4-6-8-7-5-3-1;2*1-2;;/h2*1-2,7-8H,3-6H2;2*1H3;;/q;;2*+1;2*-1/b2*2-1-,8-7-;;;;
InChI 密鑰
BGWIAAATAAWGOI-MIXQCLKLSA-N
應用
一种强大的C-H活化催化剂,用于从芳烃制备酚
用作以下反应的催化剂:
- 制备杂芳基稠合的吲哚环体系,作为HCV NS5B聚合酶的抑制剂
- 硼化反应/Suzuki-Miyaura偶联反应
- Metalation-Suzuki交叉偶联反应,用于合成联芳基和杂双芳基
- 四硼化反应
- 高度区域和对映选择性不对称硼氢化
- 通过C-H活化使芳基酮、苯甲醛和苄醇衍生物发生邻甲硅烷基化
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
A general strategy for the perfluoroalkylation of arenes and arylbromides by using arylboronate esters and [(phen) CuRF].
Litvinas N, et al.
Angewandte Chemie (International Edition in English), 51(2), 536-539 (2012)
Ir?Catalyzed Borylation of C H Bonds in N?Containing Heterocycles: Regioselectivity in the Synthesis of Heteroaryl Boronate Esters.
Mkhalid I, et al.
Angewandte Chemie (International Edition in English), 45(3), 489-491 (2006)
Direct C?H borylation and C?H arylation of pyrrolo [2, 3-d] pyrimidines: synthesis of 6, 8-disubstituted 7-deazapurines.
Klecka M, et al.
Organic & Biomolecular Chemistry, 7(5), 866-868 (2009)
Catalytic functionalization of unactivated primary C?H bonds directed by an alcohol.
Simmons E, et al.
Nature, 483(7387), 70-70 (2012)
Robert E Maleczka et al.
Journal of the American Chemical Society, 125(26), 7792-7793 (2003-06-26)
An efficient one-pot C-H activation/borylation/oxidation protocol for the preparation of phenols is described. This method is particularly attractive for the generation of meta-substituted phenols bearing ortho-/para-directing groups, as such substrates are difficult to access by other phenol syntheses.
文章
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
Ir(I)-Catalyzed C–H Borylation
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 685062-250MG | 4061833564929 |
| 685062-1G | 4061832758848 |
| 685062-25G | 4061833490624 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)