全部照片(3)
About This Item
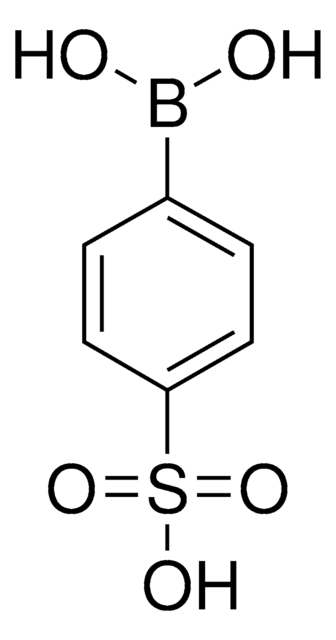
線性公式:
(H3CSO2)C6H4B(OH)2
CAS號碼:
分子量::
200.02
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
≥95.0%
形狀
solid
mp
289-293 °C
官能基
sulfone
SMILES 字串
CS(=O)(=O)c1ccc(cc1)B(O)O
InChI
1S/C7H9BO4S/c1-13(11,12)7-4-2-6(3-5-7)8(9)10/h2-5,9-10H,1H3
InChI 密鑰
VDUKDQTYMWUSAC-UHFFFAOYSA-N
一般說明
含不定量的酸酐
應用
4-(甲磺酰基)苯硼酸可作为试剂用于以下反应:
试剂用于制备
- Suzuki交叉偶联反应
- 铜催化芳基硼酸的氧化三氟甲基硫醇化反应
- 3-溴呋喃及相关杂环的定向金属化和区域选择性官能化反应
- Barton-Zard吡咯环缩合和Baeyer-Villiger氧化反应
- 二聚体环加成和钯催化的交叉偶联过程
- 用于奥当卡替中间体合成的连续流Suzuki反应
试剂用于制备
- 二芳基氨基吡啶类化合物,可作为潜在抗疟药
- 氢吡喃并吡嗪,通过氯吡嗪甲醛和烯化反应
- 联芳基砜衍生物,作为组胺H3受体的拮抗剂
- 具有潜在抗肿瘤作用的新型激酶抑制剂支架
- N-羟基异喹啉的丙型肝炎病毒抑制活性
用于 4-氧代丁烯酰胺的铑催化不对称 1,4-加成的高效硼酸。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jamie L Zigterman et al.
The Journal of organic chemistry, 72(23), 8870-8876 (2007-10-12)
A variety of 4-oxobutenamides 1 were subjected to rhodium-catalyzed conjugate addition with arylboronic acids providing high regio- and enantioselectivity (97:3 to >99:1, >96% ee) and moderate to excellent yields (54-99%). The key to high selectivity is the use of sterically
Combined batch and continuous flow procedure to the chemo-enzymatic synthesis of biaryl moiety of Odanacatib.
de Oliveira Lopes R, et al.
Journal of Molecular Catalysis. B, Enzymatic, 104, 101-107 (2014)
Pamela Kassis et al.
European journal of medicinal chemistry, 46(11), 5416-5434 (2011-09-29)
We here report the synthesis and biological evaluation of new 3-[(2-indolyl)]-5-phenyl-3,5-pyridine, 3-[(2-indolyl)]-5-phenyl-2,4-pyridine and 3-[(2-indolyl)]-5-phenyl-2,6-pyrazine derivatives designed as potential CDK inhibitors. Indoles and phenyls were used to generate several substitutions of the pyridine and pyrazine rings. The synthesis included Stille or
Optimization of a novel kinase inhibitor scaffold for the dual inhibition of JAK2 and FAK kinases
Zificsak, C. A.; et al.
Bioorganic & Medicinal Chemistry, 22, 133-137 (2012)
Facile Access to 3,5-Dihalogenated Pyrazoles by Sydnone Cycloaddition and their Versatile Functionalization by Pd-Catalyzed Cross-Coupling Processes
Delaunay, T.; et al.
European Journal of Medicinal Chemistry, 20-21, 3837-3848 (2011)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務