全部照片(1)
About This Item
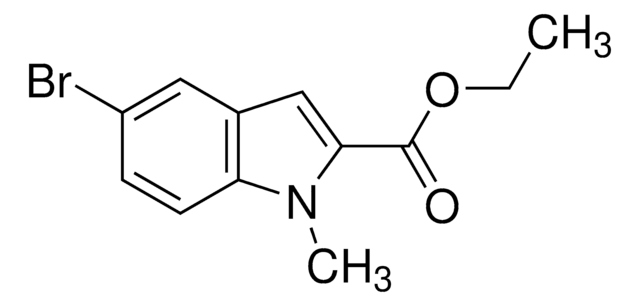
經驗公式(希爾表示法):
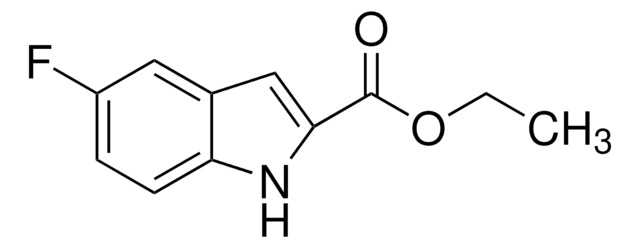
C12H13NO2
CAS號碼:
分子量::
203.24
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
形狀
solid
mp
160-164 °C
官能基
ester
SMILES 字串
CCOC(=O)c1cc2cc(C)ccc2[nH]1
InChI
1S/C12H13NO2/c1-3-15-12(14)11-7-9-6-8(2)4-5-10(9)13-11/h4-7,13H,3H2,1-2H3
InChI 密鑰
KMVFKXFOPNKHEM-UHFFFAOYSA-N
一般說明
Ethyl 5-methylindole-2-carboxylate (5-Methylindole-2-carboxylic acid ethyl ester) is an indole derivative. Indole ring system is an important building block or intermediate in the synthesis of many pharmaceutical agents. It is formed during the Fischer indolization of ethyl pyruvate 2-[2-(methanesulfonyloxy)-4-methyl]phenylhydrazine.
應用
- Reactant for synthesis of oxazino[4,3-a]indoles via cascade addition-cyclization reactions
- Reactant for preparation of indolecarboxamides as cannabinoid CB1 receptor antagonists
- Reactant for preparation of indole-3-propionic acids as antiinflammatory and analgesic agents
- Reactant for Friedel-Crafts acylation with nitrobenzoyl chloride
- Reactant for oximation reactions
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Substituent Effects in the Fischer Indolization of (2-Sulfonyloxyphenyl) hydrazones (Fischer Indolization and Related Compounds. XXX).
Murakami Y, et al.
Chemical & Pharmaceutical Bulletin, 47, 791-797 (1999)
文章
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務