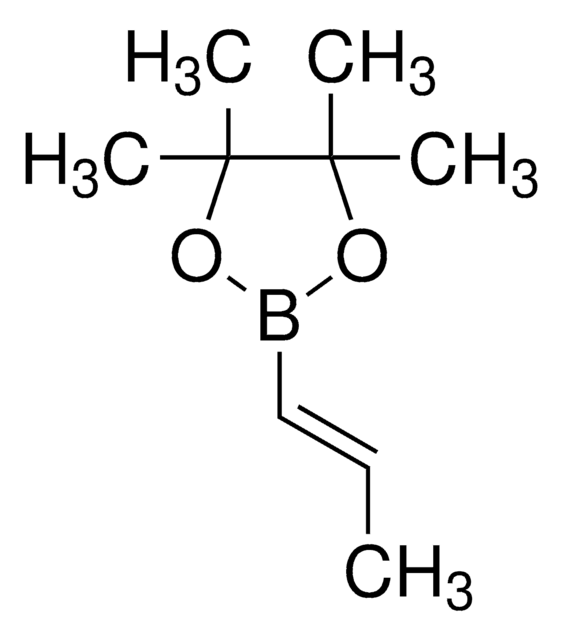
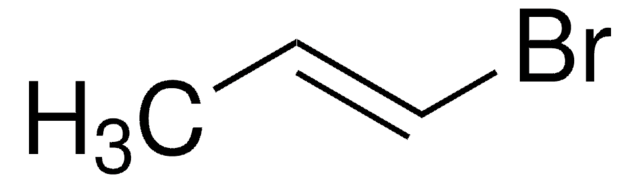
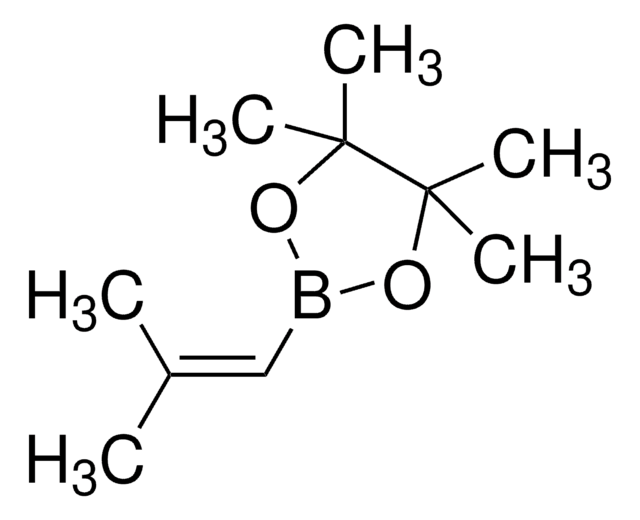
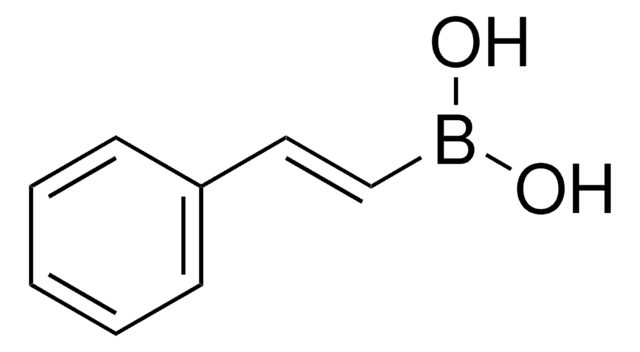
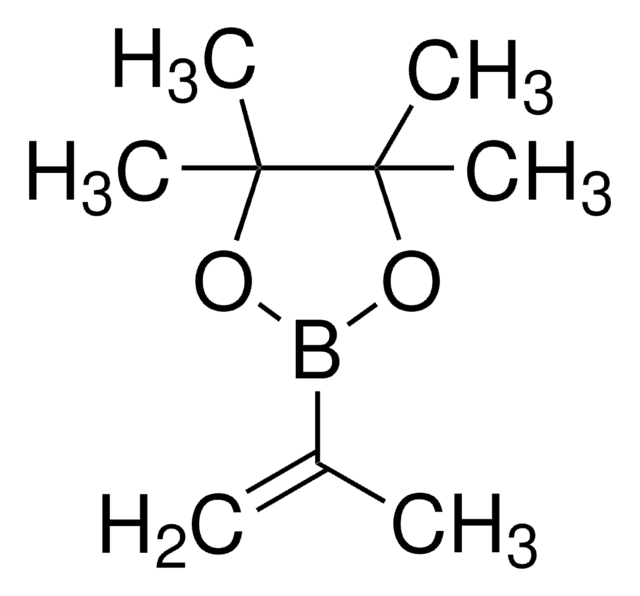
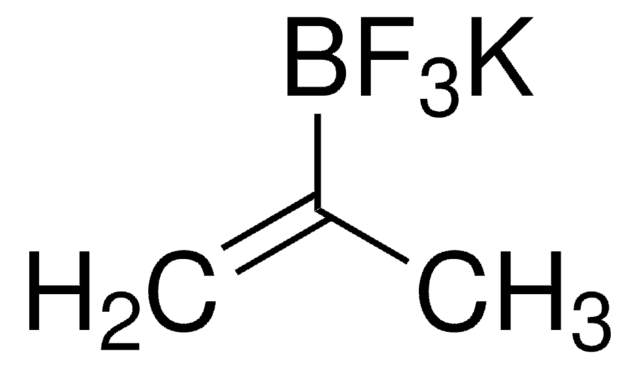
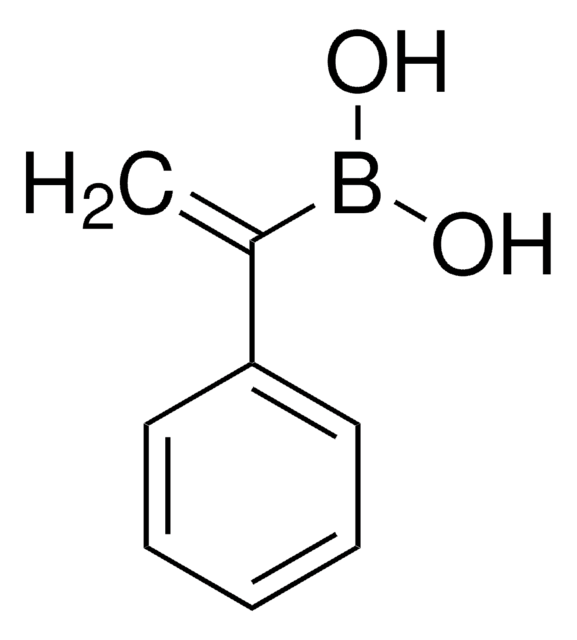
推薦產品
品質等級
化驗
≥95.0%
雜質
~10 wt. % cis-isomer
mp
123-127 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
[H]\C(C)=C(\[H])B(O)O
InChI
1S/C3H7BO2/c1-2-3-4(5)6/h2-3,5-6H,1H3/b3-2+
InChI 密鑰
CBMCZKMIOZYAHS-NSCUHMNNSA-N
應用
用于以下反应的反应物:
作为反应物用于制备:
- 钯膦催化的Suzuki-Miyaura偶联反应
- Cu(II)介导的Ullmann偶联反应
作为反应物用于制备:
- 炔基苯氧基乙酸,作为DP2受体拮抗剂,用于治疗过敏性炎症
- 四氢苯并噻吩类化合物,通过涉及Suzuki偶联的Paal-Knorr合成,作为II型脱氢喹啉酶的构象限制烯醇模拟抑制剂
- 通过二碘化钐介导的环化反应,生成高度取代的苯并环辛醇衍生物
- 通过镍催化的与炔烃和烯酮的三组分还原偶联反应,生成立体定向性二烯
用作以下反应的反应物:
作为反应物用于制备:
- 钯膦催化的Suzuki-Miyaura偶联反应
- Cu(II)介导的Ullmann偶联反应
- 钯催化的Sonogashira交叉偶联反应
作为反应物用于制备:
- 炔基苯氧基乙酸,作为DP2受体拮抗剂,用于治疗过敏性炎症
- 四氢苯并噻吩类化合物,通过涉及Suzuki偶联的Paal-Knorr合成,作为II型脱氢喹啉酶的构象限制烯醇模拟抑制剂
- 通过二碘化钐介导的环化反应,生成高度取代的苯并环辛醇衍生物
- 通过镍催化的与炔烃和烯酮的三组分还原偶联反应,生成立体定向性二烯
其他說明
含有不定量的酸酐
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Tetrahydrobenzothiophene derivatives: conformationally restricted inhibitors of type II dehydroquinase.
Sonia Paz et al.
ChemMedChem, 6(2), 266-272 (2011-01-29)
The preparation of substituted pyrazoles from β,β-dibromo-enones by a tandem condensation/Suzuki-Miyaura cross-coupling process
Beltran-Rodil, S.; et al.
Synlett, 4, 602-606 (2010)
Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-induced cyclizations.
Jakub Saadi et al.
Beilstein journal of organic chemistry, 6, 1229-1245 (2011-02-02)
A series of γ-oxo esters suitably substituted with various styrene subunits was subjected to samarium diiodide-induced 8-endo-trig cyclizations. Efficacy, regioselectivity and stereoselectivity of these reactions via samarium ketyls strongly depend on the substitution pattern of the attacked alkene moiety. The
A new strategy for the synthesis of substituted dihydropyrones and tetrahydropyrones via palladium-catalyzed coupling of thioesters
Fuwa, H.; et al.
Tetrahedron, 67, 4995-5010 (2011)
Ming-Bo Zhou et al.
The Journal of organic chemistry, 75(16), 5635-5642 (2010-08-14)
Palladium-catalyzed cross-coupling reaction of terminal alkynes with arylboronic acids has been described. In the presence of Pd(OAc)(2) and Ag(2)O, a variety of terminal alkynes, including electron-poor terminal alkynes, smoothly underwent the reaction with numerous boronic acids to afford the corresponding
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務