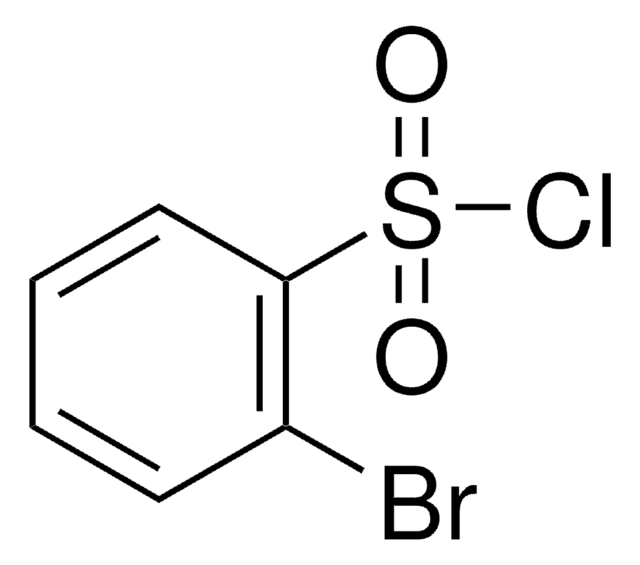
推薦產品
品質等級
化驗
96%
折射率
n20/D 1.593 (lit.)
bp
90-91 °C/0.5 mmHg (lit.)
mp
30-33 °C
密度
1.773 g/mL at 25 °C (lit.)
官能基
bromo
SMILES 字串
ClS(=O)(=O)c1cccc(Br)c1
InChI
1S/C6H4BrClO2S/c7-5-2-1-3-6(4-5)11(8,9)10/h1-4H
InChI 密鑰
PJGOLCXVWIYXRQ-UHFFFAOYSA-N
一般說明
3-Bromobenzenesulfonyl chloride is an aryl sulfonyl chloride derivative. It participates in the synthesis of N-sulfonylanthranilic acid derivatives and potent P1′ benzenesulfonyl azacyclic urea human immunodeficiency virus (HIV) protease inhibitors.
應用
3-Bromobenzenesulfonyl chloride may be used in the preparation of:
- 2-(3-bromophenyl)-5-n-butylfuran
- 2-(3-bromophenyl)-3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran
- 3-bromo-4-(3-bromophenyl)thiophene
- 2,5-bis(3-bromophenyl)-1-methylpyrrole
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Pd?Catalysed Direct Arylation of Heteroaromatics Using (Poly) halobenzenesulfonyl Chlorides as Coupling Partners: One Step Access to (Poly) halo?Substituted Bi (hetero) aryls.
Skhiri A, et al.
European Journal of Organic Chemistry, 2015(20), 4428-4436 (2015)
Peggy P Huang et al.
Bioorganic & medicinal chemistry letters, 14(15), 4075-4078 (2004-07-01)
A series of novel azacyclic urea HIV protease inhibitors bearing a benzenesulfonamide group at P1' were synthesized utilizing a parallel synthesis method. Structural studies of early analogs bound in the enzyme active site were used to design more potent inhibitors.
Zheng Yin et al.
Journal of medicinal chemistry, 52(24), 7934-7937 (2009-12-18)
A novel class of compounds containing N-sulfonylanthranilic acid was found to specifically inhibit dengue viral polymerase. The structural requirements for inhibition and a preliminary structure-activity relationship are described. A UV cross-linking experiment was used to map the allosteric binding site
文章
Aryl Sulfonyl Chloride Derivatives
Aryl sulfonyl chloride derivatives are frequently used in parallel synthesis to synthesize sulfonamides and sulfonate linkages.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 545716-1G | 4061837804267 |
| 545716-5G | 4061837804274 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務