推薦產品
交聯
2 % cross-linked
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
標籤範圍
0.3-0.8 mmol/g Cl loading
粒徑
200-400 mesh
應用
peptide synthesis
SMILES 字串
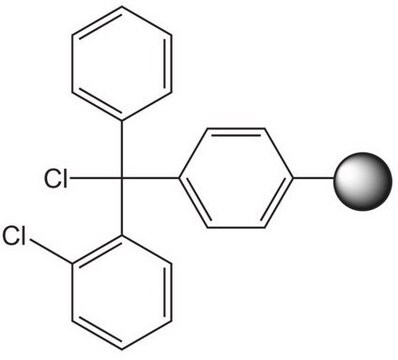
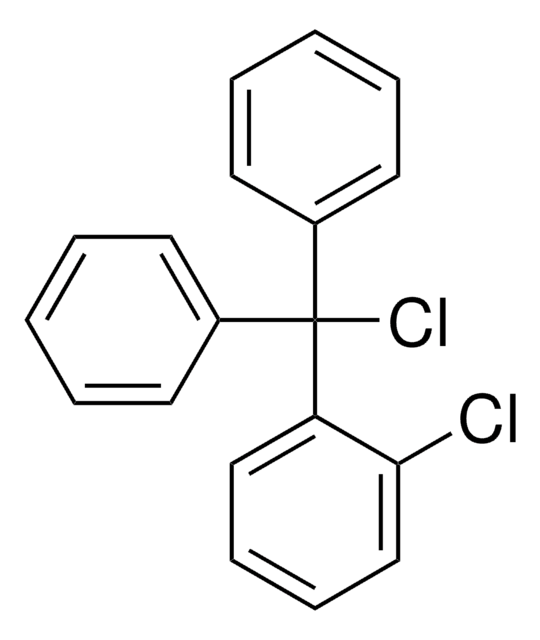
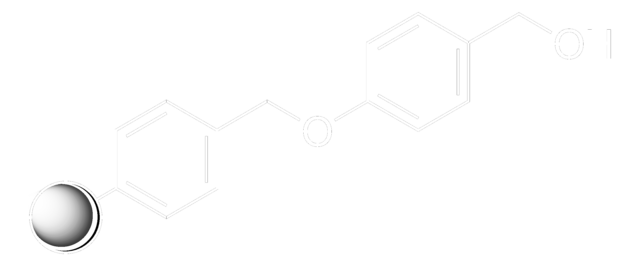
Clc1ccccc1C(Cl)(c2ccccc2)c3ccccc3
InChI
1S/C19H14Cl2/c20-18-14-8-7-13-17(18)19(21,15-9-3-1-4-10-15)16-11-5-2-6-12-16/h1-14H
InChI 密鑰
JFLSOKIMYBSASW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
- Acid labile resin used in Fmoc-based solid phase peptide synthesis.
- Mild acidic cleavage conditions lead to the release of the peptide acid where fully protected peptides can be released if desired.
- 2-Chlorotrityl chloride resins prevent racemization of the first amino acid and are thus very useful when racemic mixtures are forming (common with residues such as His or Cys).
- This resin also prevents diketopiperazide formation, which can be an issue with proline C-terminal peptide sequences.
Use:
- Attachment of the first amino acid residue is effected by stirring the resin, the protected amino acid, and excess diisopropylethylamine (DIEA) in dichloromethane.
- Cleavage of the final protected peptide fragment is achieved under very mild conditions using either acetic acid/trifluoroethanol (TFE)/dichloromethane (1:1:8; v/v/v), hexafluoroisopropanol (HFIP)/dichloromethane (1:4; v/v) or simply 0.5% trifluoroacetic acid/dichloromethane (v/v).
- Higher concentrations of TFA can be used if retention of peptide side chaing protecting groups is unimportant. Note that trityl chloride is moisture-sensitive, and, therefore, should be stored and handled appropriately.
- If the resin becomes deactivated, treatment with acetyl chloride or SOCl2 in toluene before use is recommended to restore its activity.
訊號詞
Warning
危險分類
Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務