全部照片(1)
About This Item
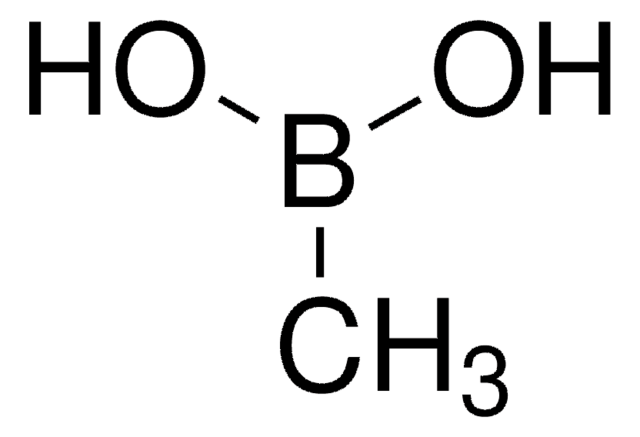
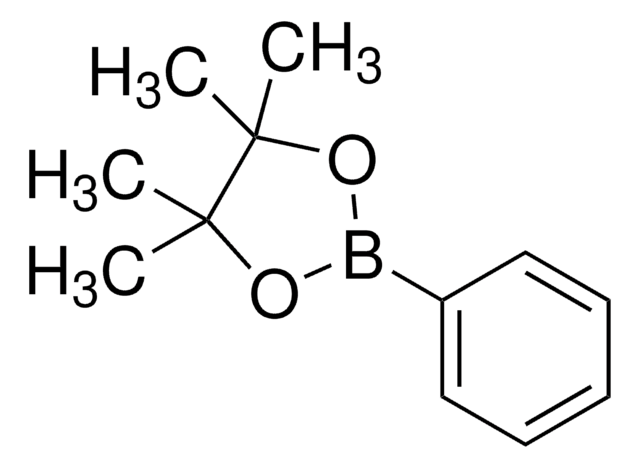
經驗公式(希爾表示法):
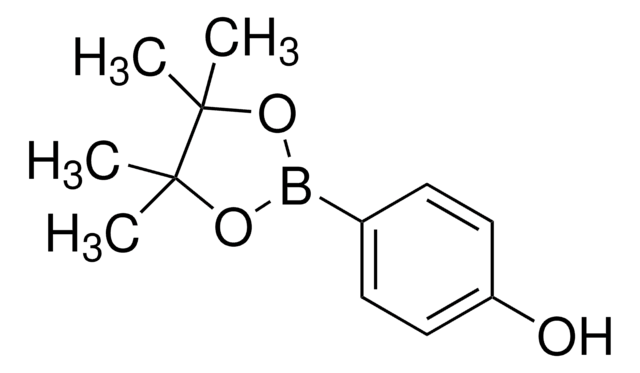
C12H17BO3
CAS號碼:
分子量::
220.07
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
98%
折射率
n20/D 1.506 (lit.)
bp
282 °C (lit.)
密度
1.05 g/mL at 25 °C (lit.)
SMILES 字串
CC1(C)OB(OC1(C)C)c2ccccc2O
InChI
1S/C12H17BO3/c1-11(2)12(3,4)16-13(15-11)9-7-5-6-8-10(9)14/h5-8,14H,1-4H3
InChI 密鑰
VLROJECCXBBKPZ-UHFFFAOYSA-N
應用
Reactant involved in:
- Synthesis of indolo-fused heterocyclic inhibitors of polymerase enzyme of hepatitis C
- Studies of pi-interactions, electronic structure and transient UV absorption of subphthalocyanine-borate-bridged ferrocene-fullerene conjugates
- Synthesis of subphthalocyanine and fused-ring nicotine derivatives
- Suzuki-Miyaura coupling-triflation sequence, reduction and salt formation for synthesis of hydroxylated oligoarene phosphines
广泛用于 Suzuki 偶联反应。
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Tetrahedron, 58, 9633-9695 (2002)
文章
The synthesis of biaryl compounds via the Suzuki coupling reaction has become more commonplace now that many arylboronic acids are readily available. We are pleased to offer arylboronic acid pinacol esters4 as part of a growing line of products used in the Suzuki coupling reaction.
Arylboronic Acid-Pinacol Esters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務