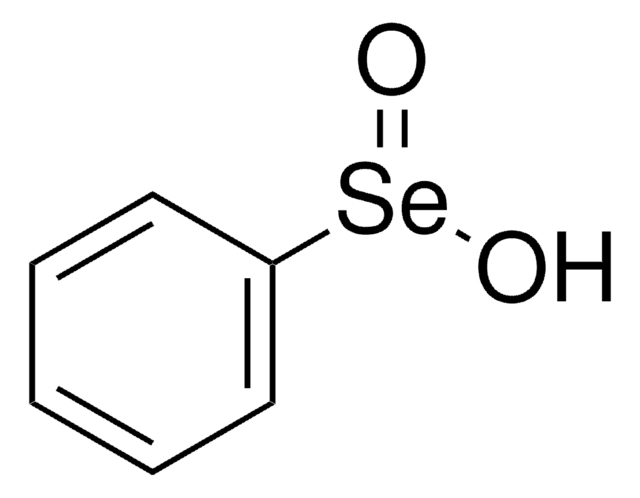
推薦產品
品質等級
化驗
96%
折射率
n20/D 1.603 (lit.)
bp
116-117 °C/12 mmHg (lit.)
密度
1.484 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
N#C[Se]c1ccccc1
InChI
1S/C7H5NSe/c8-6-9-7-4-2-1-3-5-7/h1-5H
InChI 密鑰
NODWRXQVQYOJGN-UHFFFAOYSA-N
一般說明
Phenyl selenocyanate is a selenenylation agent that can be prepared by reacting benzeneselenenyl chloride and trimethylsilyl cyanide.
應用
Phenyl selenocyanate may be used in the synthesis of:
- benzeneselenol esters
- 2-bromoethyl phenyl selenie dibromide
- α,α-dioxy-β-phenylseleno carbonitriles
- β-alkoxyalkyl phenyl selenide
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
228.2 °F - closed cup
閃點(°C)
109 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Cyanoselenenylation of ketene acetals. Synthesis of carbonyl-protected a-oxo carbonitriles.
Tomoda S, et al.
Chemistry Letters (Jpn), 11(11), 1733-1734 (1982)
A convenient synthesis of phenyl selenocyanate.
Tomoda S, et al.
Chemistry Letters (Jpn), 10(8), 1069-1070 (1981)
Aryl selenocyanates and aryl thiocyanates: reagents for the preparation of activated esters.
Grieco PA, et al.
The Journal of Organic Chemistry, 43(6), 1283-1285 (1978)
Synthesis of Aryl 2-Haloethyl Selenides and their Reactions with Potassium Selenocyanate.
Lindgren B.
Acta Chemica Scandinavica. Series B, 31(1), 1-6 (1977)
Facile oxyselenation of olefins in the presence of copper (II) or copper (I) chloride as catalyst.
Toshimitsu A, et al.
The Journal of Organic Chemistry, 45(10), 1953-1958 (1980)
文章
For microbiologists the most fundamental stain was developed in 1884 by the Danish bacteriologist Hans Christian Gram.
For microbiologists the most fundamental stain was developed in 1884 by the Danish bacteriologist Hans Christian Gram.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務