全部照片(3)
About This Item
經驗公式(希爾表示法):
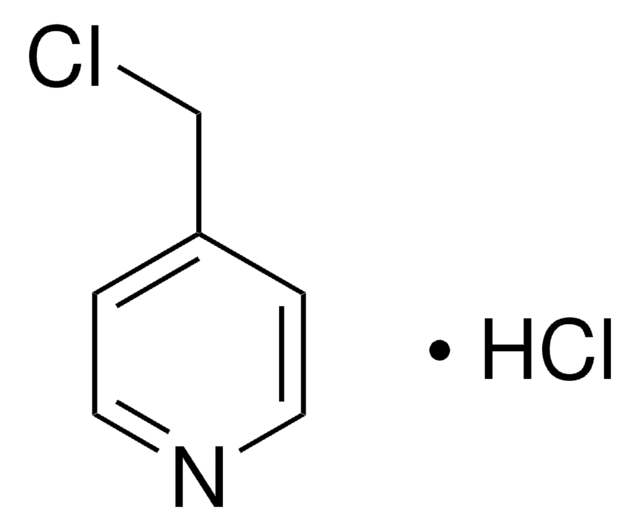
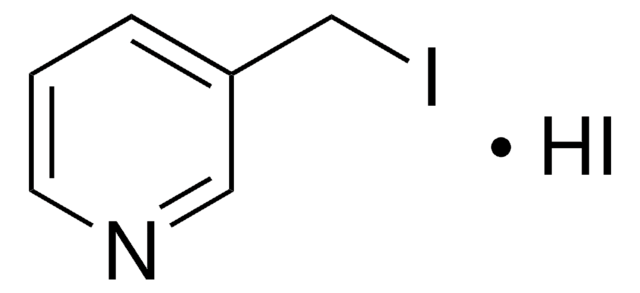
C6H6BrN · HBr
CAS號碼:
分子量::
252.93
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
mp
189-192 °C (lit.)
官能基
bromo
SMILES 字串
Br[H].BrCc1ccncc1
InChI
1S/C6H6BrN.BrH/c7-5-6-1-3-8-4-2-6;/h1-4H,5H2;1H
InChI 密鑰
VAJUUDUWDNCECT-UHFFFAOYSA-N
一般說明
4-(溴甲基)吡啶氢溴酸盐是取代的吡啶。它与 1,2-乙二胺和 1,3-丙二胺反应生成相应的二胺。
應用
4-(溴甲基)吡啶氢溴酸盐可用于制备:
- 3-(4-吡啶基甲基)-2′,3′-二-O-油基-5′-O-(4,4′-二甲氧基三苯基甲基)尿苷
- 3-(4-吡啶基甲基)-3′-O-油基-5′-O-(4,4-二甲氧基三苯甲基)-胸苷
- 1,4-双(N-己基-4-吡啶)丁二烯二氯酸盐
- 2-吗啉-4-基-7-(吡啶-4-基甲氧基)-4H-1,3-苯并恶嗪-4-酮
- 8-甲基-2-吗啉-4-基-7-(吡啶-4-基甲氧基)-4H-1,3-苯并恶嗪-4-酮
- 2-吗啉-4-基-8-(吡啶-4-基甲氧基)-4H-1,3-苯并恶嗪-4-酮
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Luca Simeone et al.
Molecular bioSystems, 7(11), 3075-3086 (2011-09-08)
Novel thymidine- or uridine-based nucleolipids, containing one hydrophilic oligo(ethylene glycol) chain and one or two oleic acid residues (called ToThy, HoThy and DoHu), have been synthesized with the aim to develop bio-compatible nanocarriers for drug delivery and/or produce pro-drugs. Microstructural
Photoinduced electron transfer in supramolecular complexes of a p-extended viologen with porphyrin monomer and dimer.
Fukuzumi S, et al.
Royal Society of Chemistry Advances, 2(9), 3741-3747 (2012)
Cristiane F da Costa et al.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 63(1), 40-42 (2008-02-12)
We report in this work the preparation and the in vitro antileishmanial activity of a series of long chains N-monoalkylated diamines and two pyridinediamine derivatives. Several compounds, tested for their in vitro antiproliferative activity against Leishmania amazonensis and Leishmania chagasi
Saleh Ihmaid et al.
European journal of medicinal chemistry, 45(11), 4934-4946 (2010-08-31)
A number of new 2-amino-[5, 6, 7 and 8]-O-substituted 1,3-benzoxazines, and 2-amino 8-methyl-7-O-substituted-1,3-benzoxazines were synthesized. Thirty one new compounds were tested for their effect on collagen induced platelet aggregation and it was found that the most active compounds were 8-methyl-2-morpholin-4-yl-7-(pyridin-3-ylmethoxy)-4H-1,3-benzoxazin-4-one
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務