全部照片(1)
About This Item
經驗公式(希爾表示法):
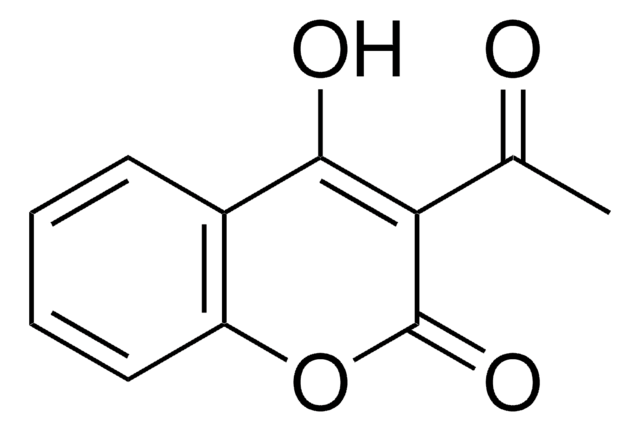
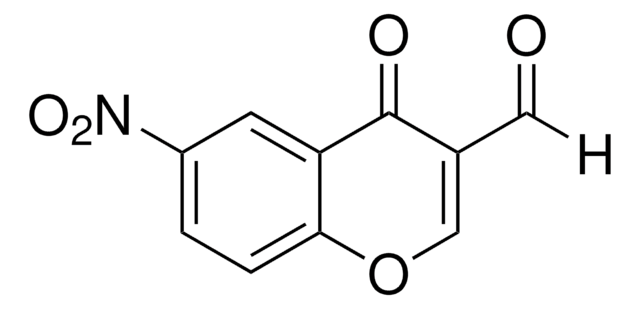
C9H5NO5
CAS號碼:
分子量::
207.14
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
98%
mp
172 °C (dec.) (lit.)
SMILES 字串
OC1=C(C(=O)Oc2ccccc12)[N+]([O-])=O
InChI
1S/C9H5NO5/c11-8-5-3-1-2-4-6(5)15-9(12)7(8)10(13)14/h1-4,11H
InChI 密鑰
NZQAQAUWFHMVEM-UHFFFAOYSA-N
一般說明
4-Hydroxy-3-nitrocoumarin is a coumarin derivative and its cytotoxic action against cultured human tumor and normal cells has been investigated. It can be prepared by the nitration of 4-hydroxycoumarin in glacial acetic acid by using 72% HNO3.
應用
4-Hydroxy-3-nitrocoumarin may be used as starting reagent for the synthesis of following compounds:
- 4-chloro-3-nitrocoumarin
- 2-unsubstituted 3-nitrochromone
- 4-amino-3-nitrocoumarins
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Biljana R Dekić et al.
Molecules (Basel, Switzerland), 15(4), 2246-2256 (2010-04-30)
Synthesis, spectral analysis and bioactivity of new coumarin derivatives are described in this paper. Eight new coumarin derivatives were synthesized in moderate to good yields by condensation of 4-chloro-3-nitrocoumarin and the corresponding heteroarylamine. The synthesized compounds were tested for their
Masami Kawase et al.
In vivo (Athens, Greece), 19(4), 705-711 (2005-07-08)
A preliminary exploration of coumarin derivatives as novel multidrug resistance (MDR) modulators was carried out to determine the basic features of the structure responsible for the MDR reversal activity. Among 44 coumarins, 14 compounds moderately induced the reversal of MDR
Vidoslav Dekić et al.
Magnetic resonance in chemistry : MRC, 48(11), 896-902 (2010-09-08)
Herein, we describe the synthesis and complete assignment of the (1)H and (13)C NMR chemical shifts of a series of antimicrobial 4-arylamino-3-nitrocoumarin derivatives based on a combination of (1)H and (13)C NMR, (1)H-(1)H-COSY, NOESY, HSQC and HMBC experiments. Conformational effects
Synthesis of heteroannulated 3-nitro-and 3-aminopyridines by cyclocondensation of electron-rich aminoheterocycles with 3-nitrochromone.
Iaroshenko VO, et al.
Tetrahedron, 68(11), 2532-2543 (2012)
Investigations of pyrans and related compounds.
Savel'ev VL, et al.
Chemistry of Heterocyclic Compounds, 9(7), 816-820 (1973)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務