全部照片(1)
About This Item
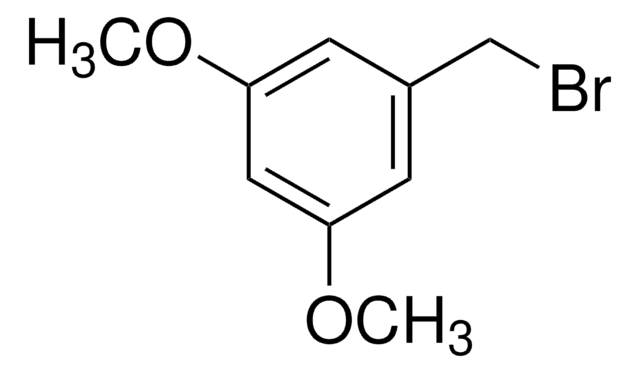
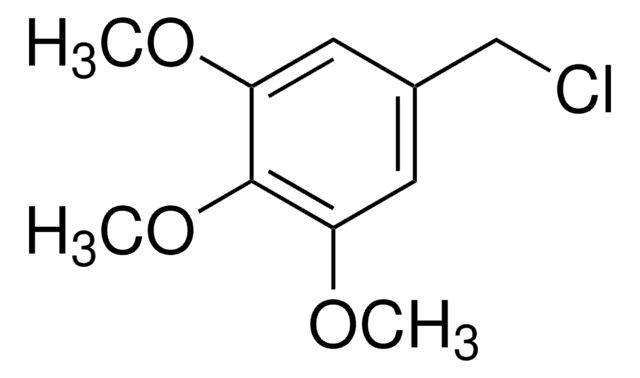
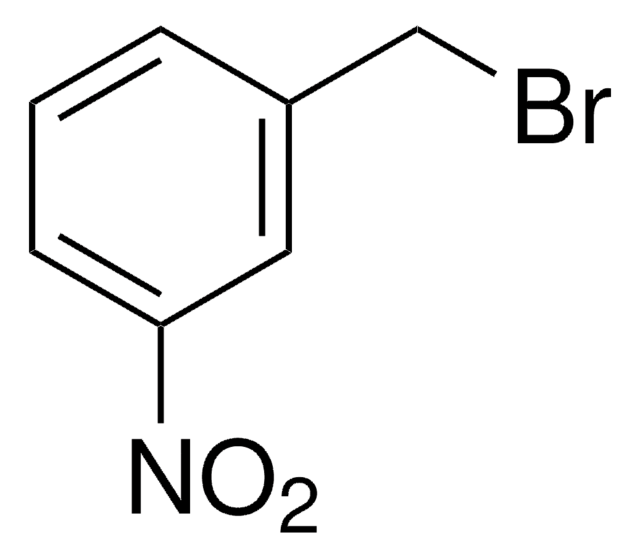
線性公式:
CH3OC6H4CH2Br
CAS號碼:
分子量::
201.06
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.575 (lit.)
bp
152 °C (lit.)
密度
1.436 g/mL at 25 °C (lit.)
SMILES 字串
COc1cccc(CBr)c1
InChI
1S/C8H9BrO/c1-10-8-4-2-3-7(5-8)6-9/h2-5H,6H2,1H3
InChI 密鑰
ZKSOJQDNSNJIQW-UHFFFAOYSA-N
一般說明
3-甲氧基苄基溴是一种苄基溴衍生物。
應用
3-甲氧基苄基溴(1-溴甲基-3-甲氧基苯)可用于手性琥珀酸衍生物的邻位二价阴离子的非对映选择性烷基化。
它可用于合成以下物质:
它可用于合成以下物质:
- 6-(3-甲氧基苯基)-己烷-2,4-二酮
- N-(3-甲氧基苄基)-N-(1-甲基-1-苯乙基)-胺
- 2-(3-甲氧基苄基)-3-[(1R)-7,7-二甲基-2-氧代双环[2.2.1]庚-1-基]-(3S)-2-硫代双环[2.2.1]-庚烷四氟硼酸盐
- 1-(3-甲氧基苄基)-5-(1-甲基-1H-咪唑-5-基)-1H-1,2,3-三唑
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Yang Zhang et al.
The Journal of organic chemistry, 71(12), 4516-4520 (2006-06-06)
A mild protocol for the conversion of beta-ketoesters and beta-diketones to carboxylic acids with use of CAN in CH3CN is described. The method is compatible with a number of functional groups, and can generate carboxylic acids under neutral conditions at
Highly diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives: a new general strategy to (R)-?-arylmethyl-?-butyrolactones.
Pohmakotr M, et al.
Tetrahedron Letters, 45(22), 4315-4318 (2004)
Synthesis of (-)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization.
Clayden J, et al.
Tetrahedron, 58(23), 4727-4733 (2002)
Application of sulfur ylide mediated epoxidations in the asymmetric synthesis of ?-hydroxy-d-lactones. Synthesis of a mevinic acid analogue and (+)-prelactone B.
Aggarwal VK, et al.
Tetrahedron Asymmetry, 60(43), 9725-9733 (2004)
Johan R Johansson et al.
The Journal of organic chemistry, 76(7), 2355-2359 (2011-03-11)
An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the primary alkyl halide
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務