推薦產品
品質等級
化驗
98%
形狀
powder
mp
>300 °C (dec.) (lit.)
SMILES 字串
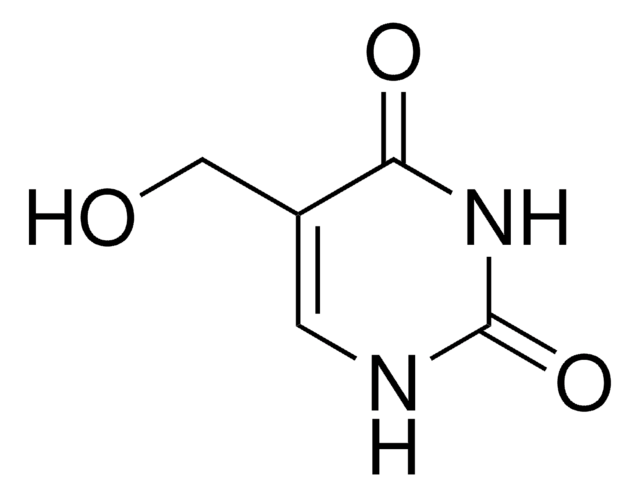
O=CC1=CNC(=O)NC1=O
InChI
1S/C5H4N2O3/c8-2-3-1-6-5(10)7-4(3)9/h1-2H,(H2,6,7,9,10)
InChI 密鑰
OHAMXGZMZZWRCA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
5-Formyluracil may be used for the preparation of covalently linked base with 5-aminocytosine pair via Schiff base formation.
訊號詞
Warning
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Q M Zhang et al.
International journal of radiation biology, 79(5), 341-349 (2003-08-29)
5-Formyluracil (5-foU) is a potentially mutagenic lesion of thymine produced in DNA by ionizing radiation and various chemical oxidants. The present authors reported previously that MutM, Nth and Nei in Escherichia coli removed 5-foU from DNA. The present study identified
Gustavo Portalone et al.
Acta crystallographica. Section C, Crystal structure communications, 63(Pt 11), o650-o654 (2007-11-09)
The asymmetric unit of the amino-oxo tautomer of 5-formyluracil (systematic name: 2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carbaldehyde), C(5)H(4)N(2)O(3), comprises one planar amino-oxo tautomer, as every atom in the structure lies on a crystallographic mirror plane. At variance with all the previously reported small-molecule crystal structures
Monica Baldini et al.
Inorganic chemistry, 42(6), 2049-2055 (2003-03-18)
Two new 5-formyluracil thiosemicarbazone (H(3)ut) derivatives, Me-H(3)ut (1) and Me(2)-H(3)ut (2), were synthesized by reacting thiosemicarbazides, mono- and dimethylated on the aminic nitrogen, with 5-formyluracil and were subsequently characterized. These ligands, treated with copper chloride and nitrate, afforded three complexes:
Chikara Dohno et al.
Journal of the American Chemical Society, 127(47), 16681-16684 (2005-11-25)
We here present a novel covalently linked base pair via Schiff base formation between 5-formyluracil (fU) and 5-aminocytosine (AmC). Formation of the Schiff base linkage proceeds reversibly and does not require any additives. The cross-linked DNA is very stable under
E J Privat et al.
Mutation research, 354(2), 151-156 (1996-07-22)
5-Formyluracil is a mutagenic base formed in DNA by oxidation of the thymine methyl group. Whereas the thymine methyl group is electron donating, the formyl group is electron withdrawing, predicting increased ionization of the N-3 imino proton under physiological conditions.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務