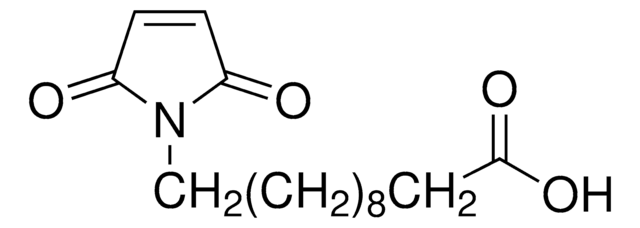
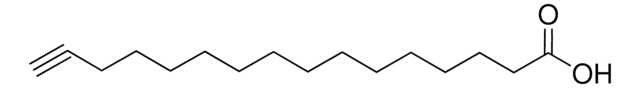
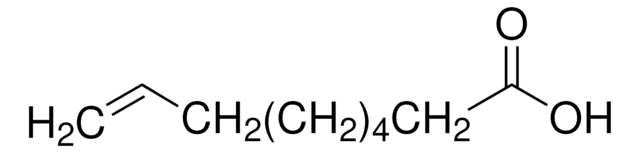推薦產品
品質等級
化驗
95%
bp
180 °C/15 mmHg (lit.)
mp
40-42 °C (lit.)
官能基
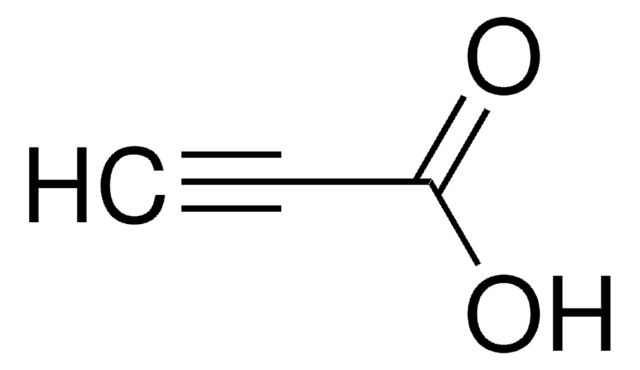
carboxylic acid
SMILES 字串
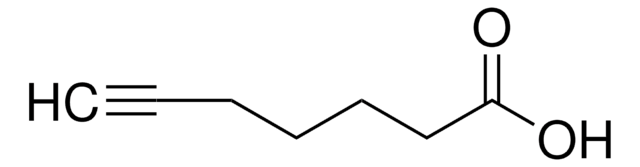
OC(=O)CCCCCCCCC#C
InChI
1S/C11H18O2/c1-2-3-4-5-6-7-8-9-10-11(12)13/h1H,3-10H2,(H,12,13)
InChI 密鑰
OAOUTNMJEFWJPO-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
10-Undecynoic acid (10- UDYA, UDY) is an acetylenic fatty acid. It is reported as highly selective irreversible inhibitor of hepatic ω- and ω-1-lauric acid hydroxylases. Enzyme catalyzed esterification of 10-undecynoic acid has been reported. UDY has been reported to be synthesized by the dehydrobromination of 10-undecenoic acid.
應用
10-Undecynoic acid was employed as model compound to investigate the microwave assisted surface click reactions catalyzed with Cu(II)/sodium L-ascorbate.†
It may be used:
It may be used:
- As a biochemical probe in an assay for the microsomal hydroxylation of lauric acid (LA), based on HPLC with flow-through radiochemical detection.
- To form molecular layers by adsorbing on the fluorite surface.
- In the supercritical hydrothermal synthesis of iron oxide nanoparticles.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
C A CaJacob et al.
Biochemistry, 25(16), 4705-4711 (1986-08-12)
The hepatic cytochrome P-450 isozymes that catalyze omega- and (omega - 1)-hydroxylation of lauric acid are specifically inactivated in vitro but not in vivo by 10-undecynoic acid. The lack of in vivo activity may result from rapid degradation of the
Gerard Lligadas et al.
Biomacromolecules, 8(6), 1858-1864 (2007-05-03)
Novel biobased aromatic triols (1,3,5-(9-hydroxynonyl)benzene and 1,3,5-(8-hydroxyoctyl)-2,4,6-octylbenzene) were synthesized through the transition-metal-catalyzed cyclotrimerization of two alkyne fatty acid methyl esters (methyl 10-undecynoate and methyl 9-octadecynoate) followed by the reduction of the ester groups to give terminal primary hydroxyl groups. A
Thiol-yne reaction of alkyne-derivatized fatty acids: biobased polyols and cytocompatibility of derived polyurethanes.
Gonzalez-Paz RJ, et al.
Polym. Chem., 3(9), 2471-2478 (2012)
Continuous hydrothermal synthesis of in situ functionalized iron oxide nanoparticles: a general strategy to produce metal oxide nanoparticles with clickable anchors.
de Tercero MD, et al.
Particle & Particle Systems Characterization, 30(3), 229-234 (2013)
C A CaJacob et al.
The Journal of biological chemistry, 263(35), 18640-18649 (1988-12-15)
Cytochrome P-450LA omega purified from clofibrate-induced rat liver oxidizes lauric acid to 11- and 12-hydroxydodecanoic acid in approximately a 1:17 ratio at a rate of 20 nmol/nmol P-450/min. In contrast, cytochrome P-450b oxidizes lauric acid much more slowly (0.5 nmol/nmol
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務