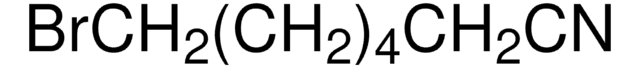推薦產品
化驗
95%
形狀
liquid
折射率
n20/D 1.477 (lit.)
bp
134 °C/13 mmHg (lit.)
密度
1.328 g/mL at 25 °C (lit.)
官能基
bromo
nitrile
SMILES 字串
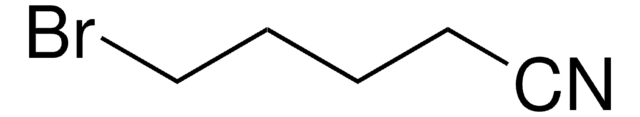
BrCCCCCC#N
InChI
1S/C6H10BrN/c7-5-3-1-2-4-6-8/h1-5H2
InChI 密鑰
PHOSWLARCIBBJZ-UHFFFAOYSA-N
一般說明
6-Bromohexanenitrile (6-Bromohexanonitrile) is an ω-bromoalkanonitrile. Friedel Crafts alkylation of 6-bromohexanenitrile with benzene has been studied.
應用
6-Bromohexanenitrile (6-Bromocapronitrile, 6-Bromohexanonitrile) is suitable reagent used in the synthesis of (5-cyanopentyl)zinc(II) bromide, an organozinc reagent. It may be used in the synthesis of the following:
- 6-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)hexanenitrile.
- dimethyl 6,6′-dithiobiscaproimidate, a long-chain dithiobisimidate.
- N-benzyloxy-(4-cyanopentyl)-carbamic acid ethyl ester, a N-benzyloxy carbamate derivative.
- 1-hetarylsulfanyl-ω-cyanoalkanes.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Friedel-Crafts alkylation of benzene by normal ?-chloroalkanoic acids and their methyl esters and nitriles.
Zakharkin LI and Anikina EV
Bulletin of the Academy of Sciences of the USSR, Division of chemical science, 36(2), 327-330 (1987)
H Peretz et al.
European journal of biochemistry, 63(1), 77-82 (1976-03-16)
This communication describes a simple method for synthesizing cleavable bifunctional imido esters of different chain lengths. These reagents, which form covalent crosslinks between lysine residues of proteins, contain a disulfide bond which is cleaved under mild conditions by reducing agents
?Green Chemical" Methods for the Regioselective Synthesis of 1-Hetarylsulfanyl-?-Cyanoalkanes."
Abele E, et al.
Latvijas Kimijas Zurnals, 49(1), 278-282 (2010)
NICKEL-CATALYZED ENANTIOSELECTIVE NEGISHI CROSS-COUPLINGS OF RACEMIC SECONDARY α-BROMO AMIDES WITH ALKYLZINC REAGENTS: (S)-N-BENZYL-7-CYANO-2-ETHYL-N-PHENYLHEPTANAMIDE.
Sha Lou et al.
Organic syntheses; an annual publication of satisfactory methods for the preparation of organic chemicals, 87, 330-330 (2010-01-01)
Yuan Liu et al.
Tetrahedron, 67(12), 2206-2214 (2011-04-19)
N-Alkyl-N-benzyloxy carbamates, 2, undergo facile intramolecular cyclization with a variety of carbon nucleophiles to give functionalized 5- and 6-membered protected cyclic hydroxamic acids, 3, in good to excellent yields. This method can be extended to prepare seven-membered cyclic hydroxamic acids
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務