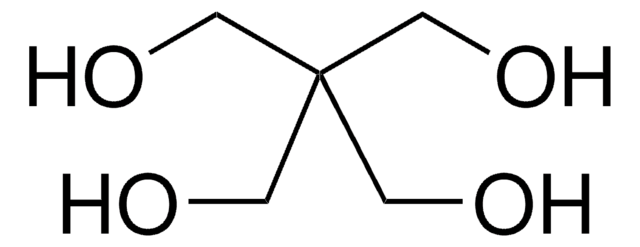
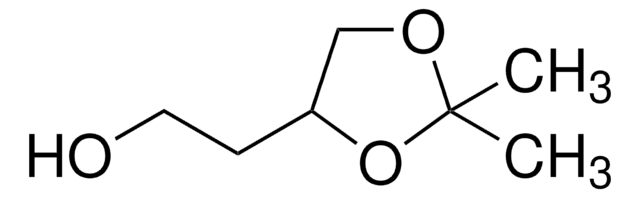
推薦產品
化驗
98%
光學活性
[α]19/D −28±2, c = 1 in methanol
光學純度
ee: 99% (GLC)
折射率
n20/D 1.475 (lit.)
bp
150 °C/0.04 mmHg (lit.)
密度
1.19 g/mL at 25 °C (lit.)
官能基
hydroxyl
SMILES 字串
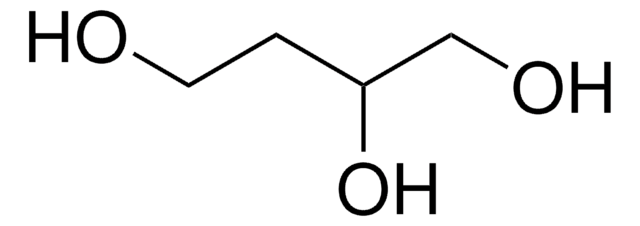
OCC[C@H](O)CO
InChI
1S/C4H10O3/c5-2-1-4(7)3-6/h4-7H,1-3H2/t4-/m0/s1
InChI 密鑰
ARXKVVRQIIOZGF-BYPYZUCNSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
(S)-(-)-1,2,4-Butanetriol can be prepared via reduction of (S)-malic acid in the presence of borane-dimethyl sulfide.
應用
(S)-(-)-1,2,4-Butanetriol may be used as a starting material in the enantioselective total syntheses of (+)-azimine and (+)-carpaine.
It can also be used to prepare the following organic building blocks:
It can also be used to prepare the following organic building blocks:
- (+)-3,4-epoxy-1-butanol
- (2S,4S)-4-(hydroxymethyl)-2-ferrocenyl-1,3-dioxane
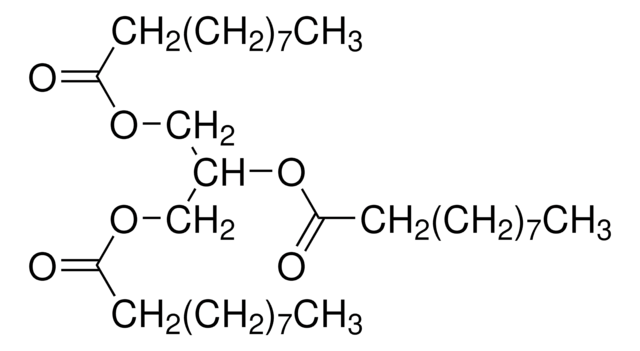
- (S)-1,2,4-triacetoxybutane via acetylation with acetic anhydride
- (S)-1,2,4-tris-(3,5-dinitrobenzoy1oxy)butane via esterification with 3,5-dinitrobenzoyl chloride
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
客戶也查看了
Facile access to (S)-1, 2, 4-butanetriol and its derivatives.
Hanessian S, et al.
Canadian Journal of Chemistry, 62(11), 2146-2147 (1984)
Asymmetric Michael addition of thiophenol to maleic acid esters
Yamashita H and Mukaiyama T
Chemistry Letters (Jpn), 14(3), 363-366 (1985)
Enantioselective total synthesis of (+)-azimine and (+)-carpaine.
Sato T, et al.
Organic Letters, 5(21), 3839-3842 (2003)
An efficient asymmetric synthesis of 2-substituted ferrocenecarboxaldehydes
Riant O, et al
The Journal of Organic Chemistry, 62(20), 6733-6745 (1997)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務