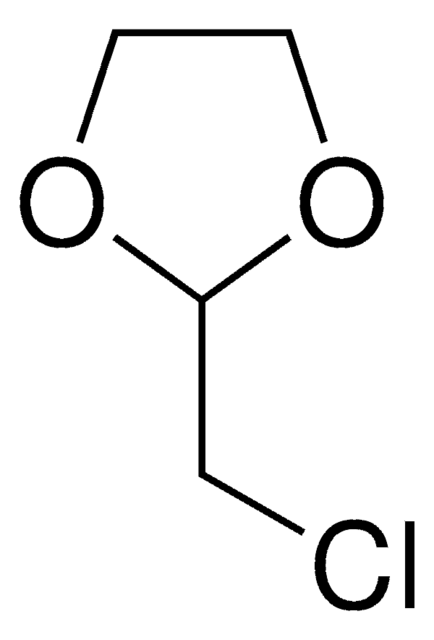
全部照片(1)
About This Item
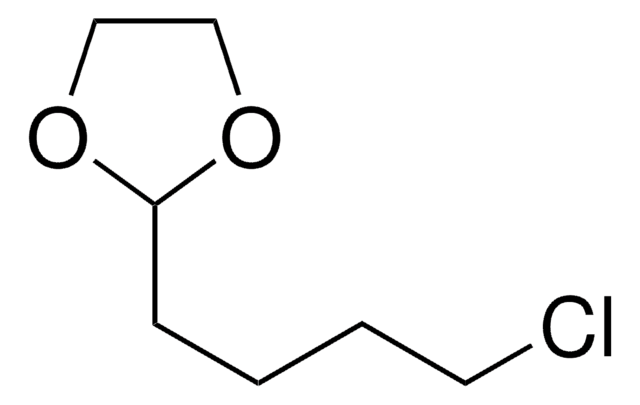
經驗公式(希爾表示法):
C6H11ClO2
CAS號碼:
分子量::
150.60
Beilstein:
1236588
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
≥97.0% (GC)
形狀
liquid
折射率
n20/D 1.453
bp
93-94 °C/12 mmHg (lit.)
密度
1.142 g/mL at 20 °C (lit.)
SMILES 字串
ClCCCC1OCCO1
InChI
1S/C6H11ClO2/c7-3-1-2-6-8-4-5-9-6/h6H,1-5H2
InChI 密鑰
ZBPUNVFDQXYNDY-UHFFFAOYSA-N
應用
2-(3-Chloropropyl)-1,3-dioxolane (2-(3′-chloropropyl)-1,3-dioxolane) is a masked γ-chlorobutyraldehyde and was used for the introduction of 3-(1,3-dioxolan-2-yl)propyl moiety. It was also used in the synthesis of:
- (±)-histrionicotoxin and (±)-histrionicotoxin 235A using a two-directional strategy
- 4-iodobutyraldehyde, 5-iodovaleraldehyde and 5-iodo-2-petanone
- corresponding phosphonate
其他說明
含掩蔽的 γ-氯丁醛,用于引入 3-(1,3-二氧戊环-2-基)丙基部分;也可用于对应膦酸酯的制备和使用
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
174.2 °F - closed cup
閃點(°C)
79 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
C.P. Forbes et al.
Journal of the Chemical Society. Perkin Transactions 1, 2353-2353 (1977)
Two-directional synthesis. Part 1: A short formal synthesis of (?)-histrionicotoxin and (?)-histrionicotoxin 235A.
Stockman RA.
Tetrahedron Letters, 41(47), 9163-9165 (2000)
S.A. Bal et al.
The Journal of Organic Chemistry, 47, 5045-5045 (1982)
R.E. Abbott et al.
The Journal of Organic Chemistry, 45, 5398-5398 (1980)
A Nagy et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2464-2469 (1996-03-19)
A convenient, high yield conversion of doxorubicin to 3'-deamino-3'-(2''-pyrroline-1''-yl)doxorubicin is described. This daunosamine-modified analog of doxorubicin is 500-1000 times more active in vitro than doxorubicin. The conversion is effected by using a 30-fold excess of 4-iodobutyraldehyde in anhydrous dimethylformamide. The
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務