全部照片(1)
About This Item
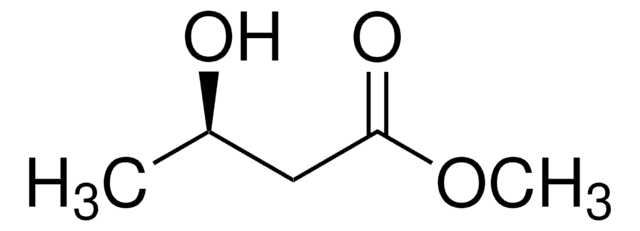
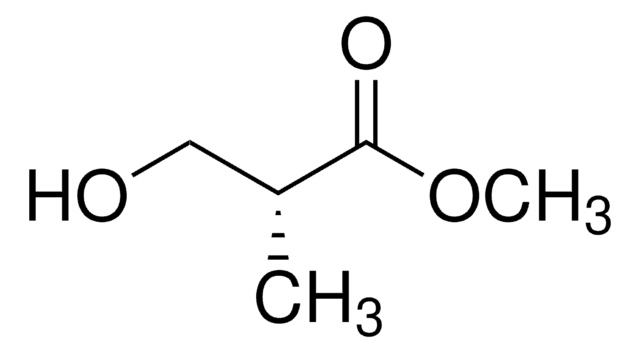
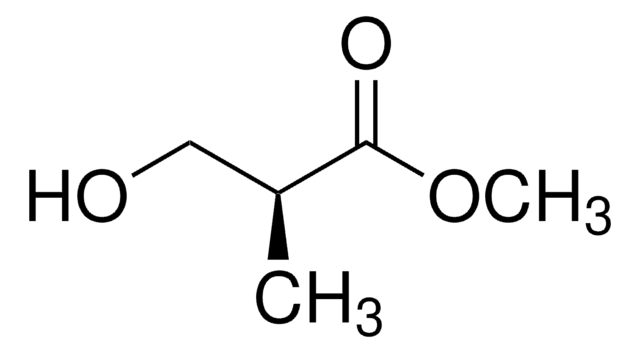
線性公式:
CH3CH(OH)CH2CO2CH3
CAS號碼:
分子量::
118.13
Beilstein:
6367546
EC號碼:
MDL號碼:
分類程式碼代碼:
12352108
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
99%
形狀
liquid
光學活性
[α]20/D +19.8°, neat
光學純度
ee: 98% (GLC)
折射率
n20/D 1.421 (lit.)
bp
63 °C/10 mmHg (lit.)
密度
1.071 g/mL at 25 °C (lit.)
官能基
ester
hydroxyl
SMILES 字串
COC(=O)C[C@H](C)O
InChI
1S/C5H10O3/c1-4(6)3-5(7)8-2/h4,6H,3H2,1-2H3/t4-/m0/s1
InChI 密鑰
LDLDJEAVRNAEBW-BYPYZUCNSA-N
應用
Methyl (S)-(+)-3-hydroxybutyrate may be used as an intermediate in the synthesis of (-)-methyl elenolate.
Optically active starting material
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
163.4 °F - closed cup
閃點(°C)
73 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
A new route to substituted 3-methoxycarbonyldihydropyrans; enantioselective synthesis of (-)-methyl elenolate.
Hatakeyama S, et al.
Journal of the Chemical Society. Chemical Communications, 17, 1202-1204 (1988)
Xiao-Hong Chen et al.
PloS one, 9(4), e94543-e94543 (2014-04-18)
A novel carbonyl reductase (AcCR) catalyzing the asymmetric reduction of ketones to enantiopure alcohols with anti-Prelog stereoselectivity was found in Acetobacter sp. CCTCC M209061 and enriched 27.5-fold with an overall yield of 0.4% by purification. The enzyme showed a homotetrameric
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務