推薦產品
蒸汽密度
5.4 (vs air)
品質等級
蒸汽壓力
<1 mmHg ( 25 °C)
化驗
98%
形狀
liquid
包含
700-1000 ppm monomethyl ether hydroquinone as inhibitor
折射率
n20/D 1.439 (lit.)
bp
182-192 °C (lit.)
密度
0.933 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
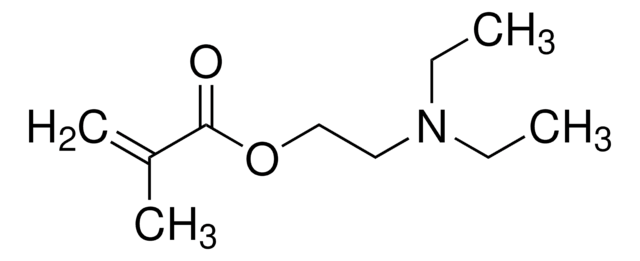
SMILES 字串
CN(C)CCOC(=O)C(C)=C
InChI
1S/C8H15NO2/c1-7(2)8(10)11-6-5-9(3)4/h1,5-6H2,2-4H3
InChI 密鑰
JKNCOURZONDCGV-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
甲基丙烯酸 2-(二甲氨基)乙酯 (DMAEMA) 是一种甲基丙烯酸衍生物,其可用作生产具有广泛应用的聚合物的单体。DMAEMA最常见的用途是生产阳离子聚合物,这种聚合物具有高电荷,可用于絮凝剂、混凝剂、分散剂和稳定剂等。此外,基于DMAEMA的聚合物由于其优异的生物相容性和生物降解性,已在药物递送系统、组织工程和基因治疗中得到应用。DMAEMA还可用作涂料、粘合剂和纺织品中的改性剂,以改善粘合性、硬度和耐水性等性能。
應用
2-(二甲胺基)甲基丙烯酸乙酯(DMAEMA)可作为起始原料用于合成聚(DMAEMA)和疏水性嵌段共聚物。聚(DMAEMA)是一种对热和pH敏感的生物相容性聚合物,广泛应用于以下应用。
- 季铵化聚(DMAEMA)可用于制备高效的抗菌磁性颗粒。通过表面引发的ATRP生成的季铵基团的高密度导致了高抗菌活性。
- 在聚(DMAEMA)刷层(brush layer)中固定的银纳米颗粒可用作表面增强拉曼光谱(SERS)检测有机分子的传感器平台。
- 它也可用于制备稳定的聚合物基基因传递系统。
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
147.2 °F - closed cup
閃點(°C)
64 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
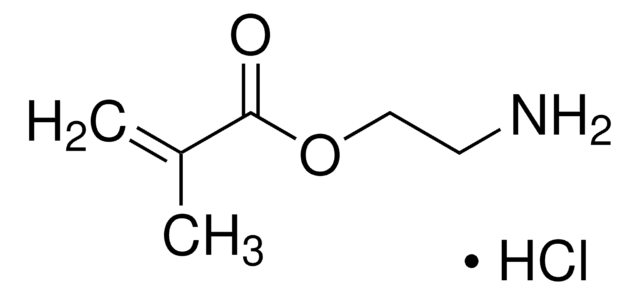
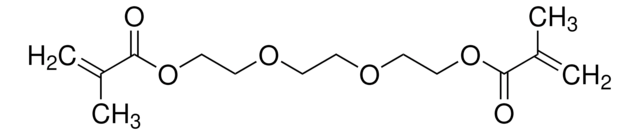
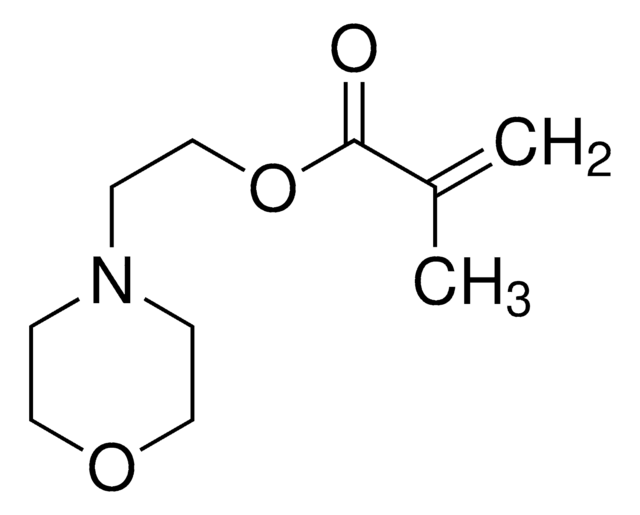
客戶也查看了
Ying Wang et al.
Biomacromolecules, 13(8), 2585-2593 (2012-07-05)
Photo- and pH-responsive amphiphilic hyperbranched star copolymers, poly(6-O-methacryloyl-1,2;3,4-di-O-isopropylidene-d-galactopyranose)[poly(2-(N,N-dimethylaminoethyl) methacrylate)-co-poly(1'-(2-methacryloxyethyl)-3',3'-dimethyl-6-nitro-spiro(2H-1-benzo-pyran-2,2'-indoline))](n)s [HPMAlpGP(PDMAEMA-co-PSPMA)(n)], were synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization of the DMAEMA and the SPMA using hyperbranched PMAlpGP as a macro RAFT agent. In aqueous solution, the copolymers self-assembled to
Hong Y Cho et al.
Biomacromolecules, 12(10), 3478-3486 (2011-09-08)
Star polymers with poly(ethylene glycol) (PEG) arms and a degradable cationic core were synthesized by the atom transfer radical copolymerization (ATRP) of poly(ethylene glycol) methyl ether methacrylate macromonomer (PEGMA), 2-(dimethylamino)ethyl methacrylate (DMAEMA), and a disulfide dimethacrylate (cross-linker, SS) via an
Synthesis of well-defined amphiphilic block copolymers with 2-(dimethylamino) ethyl methacrylate by controlled radical polymerization
Zhang Y and Matyjaszewski K
Macromolecules, 32(6), 1763-1766 (1999)
Freeze-drying of poly((2-dimethylamino)ethyl methacrylate)-based gene delivery systems.
J Y Cherng et al.
Pharmaceutical research, 14(12), 1838-1841 (1998-02-07)
Sang Beom Lee et al.
Biomacromolecules, 4(5), 1386-1393 (2003-09-10)
Amphiphilic random, gradient, and block copolymers of 2-(dimethylamino)ethyl methacrylate (DMAEMA) and n-butyl methacrylate (BMA) were synthesized by atom transfer radical polymerization (ATRP) in water/2-propanol mixtures using a methoxy-poly(ethylene glycol) (MPEG) (M(n) = 2000) macroinitiator. Kinetic studies indicate that the copolymerization
文章
The Progress in Development of Dental Restorative Materials
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![N-[(3-(二甲氨基)丙基]甲基丙烯酰胺 99%, contains MEHQ as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/295/145/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951/640/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951.png)
![[2-(甲基丙烯酰氧基)乙基] 三甲基氯化铵 溶液 75 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/316/612/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe/640/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe.png)





![[2-(甲基丙烯酰基氧基)乙基]二甲基-(3-磺酸丙基)氢氧化铵 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)