全部照片(1)
About This Item
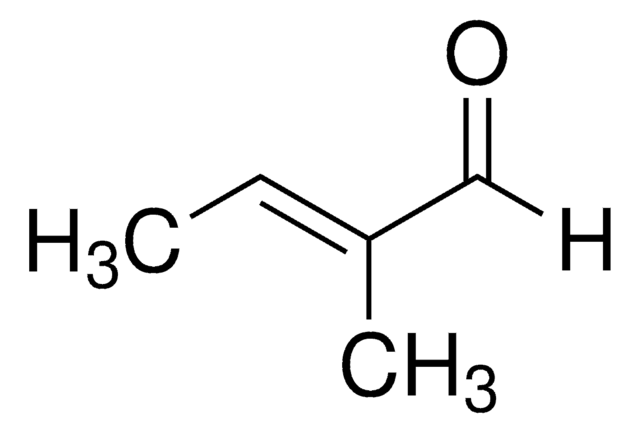
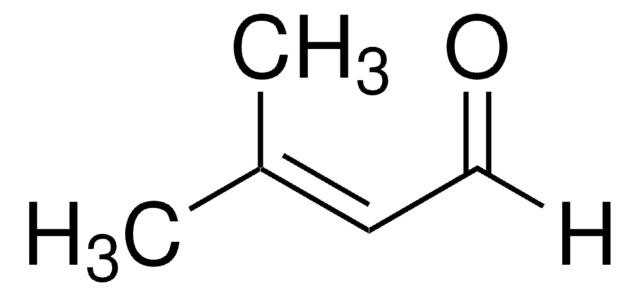
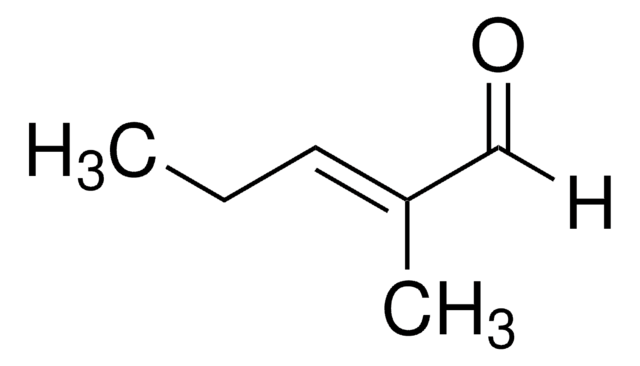
線性公式:
CH3CH=C(CH3)CHO
CAS號碼:
分子量::
84.12
Beilstein:
1698207
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
應用
噻吩醛用于 7-去甲基杀螨素 A1 的简洁全合成。它在合成过程中用作起始试剂:
- 烷基支链四烯烃,干果甲虫的信息素成分
- E,E-2,4-二甲基-2,4-六烯二烯, Leiobunum nigripalpi 的防御性分泌物的挥发性成分
- 4-甲基-2,4-己二烯-1-醇
訊號詞
Danger
危險分類
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
69.8 °F - closed cup
閃點(°C)
21 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Synthesis of nitidulid beetle pheromones: alkyl-branched tetraene hydrocarbons.
Bartelt RJ, et al.
Journal of Agricultural and Food Chemistry, 38(12), 2192-2196 (1990)
Katie A Keaton et al.
Journal of the American Chemical Society, 128(2), 408-409 (2006-01-13)
A concise total synthesis of 7-demethylpiericidin A1 has been completed. The synthesis features a titanium(II)-mediated cyclization of a (silyloxy)enyne as the key step and proceeds in nine steps from tiglic aldehyde.
Synthesis of 1, 2-and 1, 4-disubstituted tricarbonyl (pentadienyl) iron (+ 1) cations and reactions with heteroatom nucleophiles.
Donaldson WA, et al
Organometallics, 12(4), 1174-1179 (1993)
T H Jones et al.
Proceedings of the National Academy of Sciences of the United States of America, 74(2), 419-422 (1977-02-01)
Analyses of the chief volatile constituents of the defensive secretions of three oplionids were carried out. Leiobunum nigripalpi produces three closely related C7 compounds: E-4-methyl-4-hexen-3-one(I), 4-methylhexan-3-one(II), and 4-methylhexan-3-ol(III), along with E-4-methyl-4-hepten-3-one(IV), E,E-2,4-dimethylhexa-2,4-dienal(IX), and a minor, unidentified component. L. leiopenis secretion
Hasmik Grigoryan et al.
Carcinogenesis, 39(5), 661-668 (2018-03-15)
Although benzene has long been recognized as a cause of human leukemia, the mechanism by which this simple molecule causes cancer has been problematic. A complicating factor is benzene metabolism, which produces many reactive intermediates, some specific to benzene and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務