推薦產品
品質等級
化驗
99%
形狀
liquid
折射率
n20/D 1.549 (lit.)
bp
188-189 °C (lit.)
溶解度
alcohol: soluble
carbon disulfide: soluble
chloroform: soluble
diethyl ether: soluble
密度
1.065 g/mL at 25 °C (lit.)
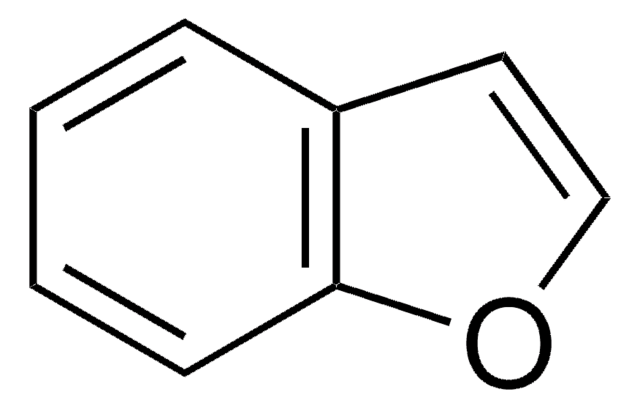
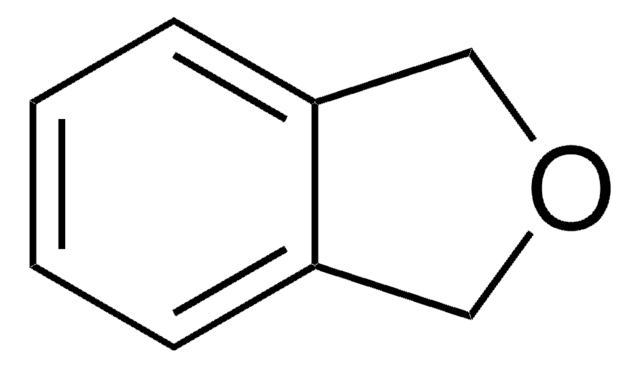
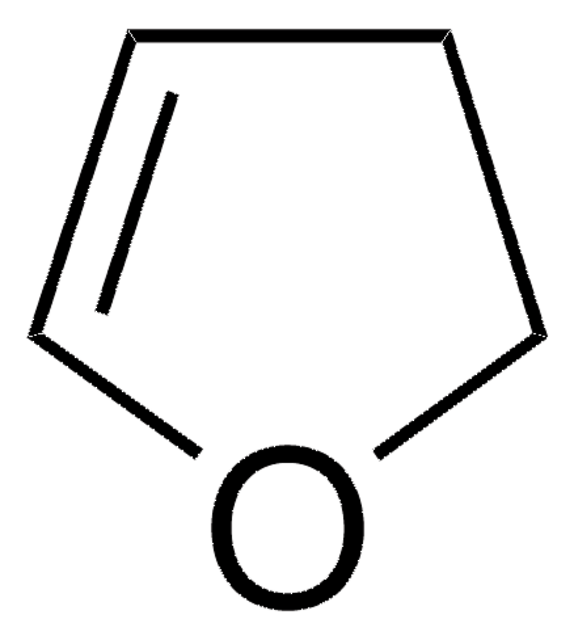
SMILES 字串
C1Cc2ccccc2O1
InChI
1S/C8H8O/c1-2-4-8-7(3-1)5-6-9-8/h1-4H,5-6H2
InChI 密鑰
HBEDSQVIWPRPAY-UHFFFAOYSA-N
基因資訊
human ... CYP1A2(1544)
尋找類似的產品? 前往 產品比較指南
一般說明
已经研究了使用恶臭假单胞菌 UV4 的完整细胞生物转化 2,3-二氢苯并呋喃。2,3-二氢苯并呋喃是在苯并呋喃的催化加氢脱氧过程中形成的中间体。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
152.6 °F - closed cup
閃點(°C)
67 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Prashant P Deshpande et al.
Journal of industrial microbiology & biotechnology, 35(8), 901-906 (2008-05-23)
Microbial hydroxylation of o-bromophenylacetic acid provided 2-bromo-5-hydroxyphenylacetic acid. This enabled a route to the key intermediate 4-bromo-2,3-dihydrobenzofuran for synthesizing a melatonin receptor agonist and sodium hydrogen exchange compounds. Pd-mediated coupling reactions of 4-bromo-2,3-dihydrobenzofuran provided easy access to the 4-substituted-2,3-dihydrobenzofurans.
Łukasz Albrecht et al.
Angewandte Chemie (International ed. in English), 50(52), 12496-12500 (2011-11-09)
Fine-tuning: Three types of optically active trans-2,3-disubstituted-2,3-dihydrobenzofurans having three contiguous stereogenic centers can be efficiently accessed by one-pot reaction cascades (see scheme; TMS = trimethylsilyl). High substitution diversity of the final products can be achieved from the same common precursors
Derrick L J Clive et al.
Chemical communications (Cambridge, England), (21)(21), 2151-2153 (2007-05-24)
(-)-Conocarpan (1) was synthesized by a method based on radical cyclization, and the absolute configuration was established by chemical degradation; the original 2R,3R-assignment to (+)-conocarpan should be reversed, as suggested by a later chiroptical study of model 2,3-dihydrobenzofurans.
Leticia Jiménez-González et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(34), 8762-8769 (2006-09-06)
2,3-Dihydrobenzofurans can be diastereoselectively prepared by condensation of aromatic aldehydes with 2,3-dihydrobenzoxasilepines under the catalysis of Ag(I) complexes, and in the presence of a source of fluoride ion. The application of this strategy by using chiral catalysts leads to a
H S Heine et al.
Chemico-biological interactions, 59(2), 219-230 (1986-09-01)
The effects of dietary administration of equimolar doses (5 mmol/kg body wt per day) of trimethylene oxide, trimethylene sulfide, coumaran, benzofuran, indole, and indole-3-carbinol on the activities of microsomal epoxide hydrolase and several other xenobiotic metabolizing enzymes were measured in
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務